��Ŀ����
![]() �ۻ�ѧ��ѡ��ѧ�뼼����
�ۻ�ѧ��ѡ��ѧ�뼼����
ͨ������£���������CO2�������������0��050��ʱ�����������Ե�����ЧӦ��Ϊ��С������CO2�Ի�����Ӱ�죬������������CO2��������ͬʱҲ��ǿ��CO2�������õ��о���
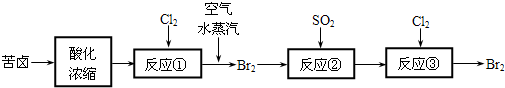
��1��Ŀǰ���ƹ��ó��ٽ�CO2(������̬��Һ̬֮��)��������������������һ�����Ի����Ļ���������___________________��
��2����ѧ��Ϊ��ȡ�����е�CO2���ѿ�������̼�����Һ��Ȼ���ٰ�CO2����Һ����ȡ����������ѧ��Ӧʹ֮��Ϊ������ȼ�ϼ״����������£�

�ٷֽ���з�Ӧ�Ļ�ѧ����ʽΪ��_____________________________��
�ںϳ����У�����4.4g CO2������H2ǡ�÷�Ӧ������̬����ų�4.947kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ��________________________��
��3��ijͬѧ���ó������ⶨ������CO2����������������CaCO3��BaCO3���ܶȻ�K(sp)�ֱ�Ϊ4��96��10-9��2��58��10-9������ý�����ͨ��������__________��Һ��ʵ��ʱ���ⶨ�¶ȡ�ѹǿ�Ϳ���������⣬����Ҫ�ⶨ_____________��
��1������������(3��)
��2����2KHCO3 ![]() K2CO3+CO2��+H2O(3��)
K2CO3+CO2��+H2O(3��)
��CO2(g)+3H2(g)��CH3OH(g)+H2O(g)����H��-49.47kJ��mol(3��)
��3��Ba(OH)2(��NaOH��BaCl2�����Һ��(3��) ���ɳ���������(3��)
����:

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д� �������ϵ�д�
�������ϵ�д���ͼ��ij�����Ժ�ˮ��Դ�����ۺ����õ�ʾ��ͼ��
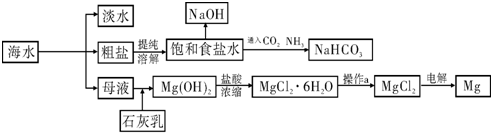
��ش��������⣺
��1�����о�һ�ֺ�ˮ�����ķ���
��2����ҵ�ϳ������ӽ���Ĥ��������NaOH���������д���ͨ�����ӽ���Ĥ��������
��3�������Ƽ��������ʳ��ˮ��ͨ��CO2��NH3�Ƶ�NaHCO3����ͨ��
���ѧʽ����������
��4��þ��һ����;�ܹ㷺�Ľ������ϣ�Ŀǰ������60%��þ�Ӻ�ˮ����ȡ����֪�й����ʵ��۷е��������£�
| MgO | MgCl2 | |
| �۵�/�� | 2852 | 714 |
| �е�/�� | 3600 | 1412 |
 2NH3��+CaCl2+2H2O
2NH3��+CaCl2+2H2O Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O