��Ŀ����
����ѧ--ѡ��ѧ�뼼������ͼ��ij�����Ժ�ˮ��Դ�����ۺ����õ�ʾ��ͼ��
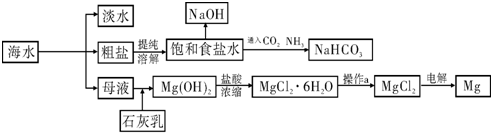
��ش��������⣺
��1�����о�һ�ֺ�ˮ�����ķ���
��2����ҵ�ϳ������ӽ���Ĥ��������NaOH���������д���ͨ�����ӽ���Ĥ��������
��3�������Ƽ��������ʳ��ˮ��ͨ��CO2��NH3�Ƶ�NaHCO3����ͨ��
���ѧʽ����������
��4��þ��һ����;�ܹ㷺�Ľ������ϣ�Ŀǰ������60%��þ�Ӻ�ˮ����ȡ����֪�й����ʵ��۷е��������£�
| MgO | MgCl2 | |
| �۵�/�� | 2852 | 714 |
| �е�/�� | 3600 | 1412 |
��������1����ˮ���������ú�ˮ����������ˮ�Ĺ��̣������ķ����У�����Ĥ�����䶳�������ӽ������ȣ�
��2�������ӽ���Ĥֻ����������ͨ�����������Ӳ���ͨ���������ϵĵ缫��ӦʽΪ2H++2e-=H2���������ӵ����ļ��٣��ƻ�ˮ�ĵ���ƽ�⣬���������������ӣ�����Ȼ�����Һ�����������������������������ƣ�
��3������ȡ̼������ʱ��Ҫ�õ������ı���ʳ��ˮ���Լ�����������ˮ�����жϣ�NaHCO3���ȷֽ�õ�����Na2CO3��
��4���ɱ���֪��MgO��MgCl2�۵�ߣ����ĵĵ��ܶࣻ
��2�������ӽ���Ĥֻ����������ͨ�����������Ӳ���ͨ���������ϵĵ缫��ӦʽΪ2H++2e-=H2���������ӵ����ļ��٣��ƻ�ˮ�ĵ���ƽ�⣬���������������ӣ�����Ȼ�����Һ�����������������������������ƣ�
��3������ȡ̼������ʱ��Ҫ�õ������ı���ʳ��ˮ���Լ�����������ˮ�����жϣ�NaHCO3���ȷֽ�õ�����Na2CO3��
��4���ɱ���֪��MgO��MgCl2�۵�ߣ����ĵĵ��ܶࣻ
����⣺��1����ˮ���������ú�ˮ����������ˮ�Ĺ��̣������ķ����У�����Ĥ�����䶳�������ӽ������ȣ��ʴ�Ϊ��������Ĥ�����䶳�������ӽ������ȣ���
��2�������ӽ���Ĥֻ����������������ͨ�������������������������Ӿ�����ͨ����
�����ϵĵ缫��ӦʽΪ��2H++2e-=H2���������ӵ����ļ��٣��ƻ�ˮ�ĵ���ƽ�⣬���������������ӣ�����NaOH����NaOH�ڵ��۵����������ɣ�
����Ȼ�����Һ�����������������������������ƣ�����ʽΪ��2NaCl+2H2O
2NaOH+H2��+Cl2 ����
�ʴ�Ϊ��Na+��������2NaCl+2H2O
2NaOH+H2��+Cl2 ����
��3����ȡ̼������ʱ��Ҫ�õ������ı���ʳ��ˮ������Ӧ��ͨ�백�������������ܽ���ˮ�����γɽϴ�Ũ�ȵ���Һ�������ڶ�����̼���գ����ɸ����̼����泥�NaHCO3���ȷֽ�õ�����Na2CO3������ʽΪ��2NaHCO3
Na2CO3+CO2��+H2O��
�ʴ�Ϊ��NH3�����������ܽ���ˮ�����γɽϴ�Ũ�ȵ���Һ�������ڶ�����̼���գ����ɸ����̼����泥�2NaHCO3
Na2CO3+CO2��+H2O��
��4��MgO��MgCl2�۵�ߣ����MgO�ȵ��MgCl2�ĵ��ܶ࣬�˷���Դ��
�ʴ�Ϊ��MgO��MgCl2�۵�ߣ����MgO�ȵ��MgCl2�ĵ��࣮ܶ
��2�������ӽ���Ĥֻ����������������ͨ�������������������������Ӿ�����ͨ����
�����ϵĵ缫��ӦʽΪ��2H++2e-=H2���������ӵ����ļ��٣��ƻ�ˮ�ĵ���ƽ�⣬���������������ӣ�����NaOH����NaOH�ڵ��۵����������ɣ�
����Ȼ�����Һ�����������������������������ƣ�����ʽΪ��2NaCl+2H2O
| ||
�ʴ�Ϊ��Na+��������2NaCl+2H2O
| ||
��3����ȡ̼������ʱ��Ҫ�õ������ı���ʳ��ˮ������Ӧ��ͨ�백�������������ܽ���ˮ�����γɽϴ�Ũ�ȵ���Һ�������ڶ�����̼���գ����ɸ����̼����泥�NaHCO3���ȷֽ�õ�����Na2CO3������ʽΪ��2NaHCO3
| ||
�ʴ�Ϊ��NH3�����������ܽ���ˮ�����γɽϴ�Ũ�ȵ���Һ�������ڶ�����̼���գ����ɸ����̼����泥�2NaHCO3
| ||
��4��MgO��MgCl2�۵�ߣ����MgO�ȵ��MgCl2�ĵ��ܶ࣬�˷���Դ��
�ʴ�Ϊ��MgO��MgCl2�۵�ߣ����MgO�ȵ��MgCl2�ĵ��࣮ܶ
������������һ���ۺ������⣬�漰֪ʶ��Ƚ϶࣬Ҫ��ѧ����������֪ʶ�ṹ�ͷ���������������Կα�֪ʶ�ܹ��ι̵����գ�

��ϰ��ϵ�д�
�����Ŀ
 2NH3��+CaCl2+2H2O
2NH3��+CaCl2+2H2O Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O