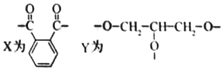��Ŀ����
����Ŀ������ϩ����Ҫ�Ļ���ԭ�ϡ���ʵ�����Ʊ��������£�

�ش��������⣺
����ϩ���Ʊ����ᴿ
(1)ԭ�ϻ������������������ʣ������Լ�Ϊ____________������Ϊ__________________��
(2)����1![]() װ����ͼ��ʾ�����Ⱥͼг�װ������ȥ����
װ����ͼ��ʾ�����Ⱥͼг�װ������ȥ����

����ƿA�н��еĿ��淴Ӧ��ѧ����ʽΪ________________________
������B������Ϊ____________��
(3)������3�����IJ��貹�룺��װ����װ�ã��������������ʺͷ�ʯ��____________����ȥǰ��֣��ռ�83�����֡�
����ϩ�����IJⶨ
��һ�������£���![]() ����ϩ��Ʒ�м��붨���Ƶõ�
����ϩ��Ʒ�м��붨���Ƶõ�![]() ���뻷��ϩ��ַ�Ӧ��ʣ���
���뻷��ϩ��ַ�Ӧ��ʣ���![]() ������
������![]() ��������
��������![]() �����ɵ�I2��
�����ɵ�I2��![]() ��
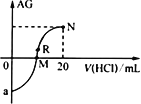
��![]() ����Һ���䷴Ӧ��ǡ������
����Һ���䷴Ӧ��ǡ������![]() ����Һ
����Һ![]() ���������ݾ��ѿ۳��������أ���
���������ݾ��ѿ۳��������أ���
�ⶨ�����У������ķ�Ӧ���£���![]()
��![]() I2+2Na2S2O3=2NaI+Na2S4O6
I2+2Na2S2O3=2NaI+Na2S4O6
(4)������Ʒ�л���ϩ����������Ϊ____________������ĸ��ʾ����
(5)��������ᵼ�²ⶨ���ƫ�͵���____________������ţ���
a����Ʒ�к��б������� b���ڲⶨ�����в��ֻ���ϩ�ӷ� c��![]() ����Һ���ֱ�����
����Һ���ֱ�����
���𰸡�FeCl3��Һ ��Һ����ɫ ![]()
![]()
![]() +H2O ���ٻ��������������������� ͨ����ˮ������
+H2O ���ٻ��������������������� ͨ����ˮ������  bc
bc
��������
������������ȥ��Ӧ���ɻ���ϩ��ˮ������ϩ������ˮ������ʳ��ˮ�ܽ�������Ȼ�����Ȼ���Һ�õ���ˮ���к��л��������Ȼ������õ����л����к��л���ϩ��Ȼ�������ˡ�����õ�����ϩ��
(1)�����ܺ��Ȼ�����Һ������ɫ��Ӧ��
(2)����ƿA�н��еĿ��淴ӦΪ����������ȥ��Ӧ��
������B������������������
(3)������3�����IJ��貹�룺��װ����װ�ã��������������ʺͷ�ʯ��ͨ����ˮ�����ȣ���ȥǰ��֣��ռ�83�����֣�
(4)�������ĵ�Na2S2O3���������Br2�����Br2�����������뻷��ϩ��Ӧ��Br2����һ��ȷ������ϩ�����ʵ�����Ȼ����m=nM������Ʒ�л���ϩ������������
(5) a. ��Ʒ�к��б������ʣ����²ⶨ���ƫ�ߣ�
b. �ڲⶨ�����в��ֻ���ϩ�ӷ���������Ʒ���ĵ�Br2�����ʵ���ƫС�����ĵ�Na2S2O3��Һ���ƫ������ƫ�ͣ�
c. Na2S2O3����Һ���ֱ����������µζ�I2ʱ��ʹ��Na2S2O3����Һ��������ӣ���ʣ��Br2�����ʵ�������ƫ������Br2�����ʵ���ƫС��������ƫ�͡�
������������ȥ��Ӧ���ɻ���ϩ��ˮ������ϩ������ˮ������ʳ��ˮ�ܽ�������Ȼ�����Ȼ���Һ�õ���ˮ���к��л��������Ȼ������õ����л����к��л���ϩ��Ȼ�������ˡ�����õ�����ϩ��
(1)�����ܺ��Ȼ�����Һ������ɫ��Ӧ�����Կ�����FeCl3��Һ���鱽�ӣ����Ӻ��Ȼ�����Һ�����Һ����ɫ���ʴ�Ϊ��FeCl3��Һ����Һ����ɫ��
(2)����ƿA�л�����������ȥ��Ӧ���ɻ���ϩ��ˮ���÷�Ӧ����ʽΪ![]()
![]()
![]() +H2O��
+H2O��
������B����������������������B�������Ǽ��ٻ����������������������ʣ��ʴ�Ϊ�����ٻ���������(����������)��
(3)������3(����)�IJ��貹�룺��װ����װ�ã��������������ʺͷ�ʯ��ͨ����ˮ�����ȣ���ȥǰ��֣��ռ�83�����֣��ʴ�Ϊ��ͨ����ˮ�����ȣ�
(4)��һ�������£���a g����ϩ��Ʒ�м���b molBr2���뻷��ϩ��ַ�Ӧ��ʣ���Br2������KI��Ӧ����I2����Na2S2O3�ζ�I2�����ݷ�ӦBr2+2KI�TI2+2KBr��I2+2Na2S2O3=2NaI+Na2S2O4�ó���ϵʽBr2~I2~2Na2S2O3�����뻷��ϩ��Ӧ��ʣ���Br2�����ʵ���Ϊ![]() mol���뻷��ϩ��Ӧ��Br2�����ʵ���Ϊ��b-
mol���뻷��ϩ��Ӧ��Br2�����ʵ���Ϊ��b-![]() ��mol������
��mol������![]() ��n(����ϩ)= ��b-
��n(����ϩ)= ��b-![]() ��mol��������Ʒ�л���ϩ����������Ϊ��
��mol��������Ʒ�л���ϩ����������Ϊ��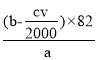 ���ʴ�Ϊ��
���ʴ�Ϊ��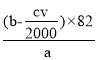 ��
��
(5)a. ��Ʒ�к��б������ʣ�����Ҳ���巴Ӧ�����²ⶨ���ƫ�ߣ�
b. �ڲⶨ�����в��ֻ���ϩ�ӷ���������Ʒ���ĵ�Br2�����ʵ���ƫС�����ĵ�Na2S2O3��Һ���ƫ������ƫ�ͣ�
c. Na2S2O3����Һ���ֱ����������µζ�I2ʱ��ʹ��Na2S2O3����Һ��������ӣ���ʣ��Br2�����ʵ���ƫ������Br2�����ʵ���ƫС��������ƫ�ͣ�
�ʴ�Ϊ��bc��