��Ŀ����
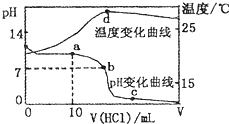 �����£���1.00mol?L-1�������20.00 mL 1.00mol?L-1�İ�ˮ�У���ҺpH���¶��������������ı仯������ͼ��ʾ�������й�˵���в���ȷ���ǣ�������
�����£���1.00mol?L-1�������20.00 mL 1.00mol?L-1�İ�ˮ�У���ҺpH���¶��������������ı仯������ͼ��ʾ�������й�˵���в���ȷ���ǣ�������A��a����Һ������Ũ�ȴ�С�Ĺ�ϵ��c��NH
| ||
B��b����Һ������Ũ�ȴ�С�Ĺ�ϵ��c��NH
| ||
C��c����Һ������Ũ�ȴ�С�Ĺ�ϵ��c��NH
| ||
| D��d��ʱ��Һ�¶ȴﵽ��ߣ�֮���¶������½���ԭ����NH3?H2O�������� |
������A��a�������10mL���ᣬ���ݷ�Ӧ�����Һ����ɷ���������Ũ�ȴ�С��
B��b��pH=7����c��H+��=c��OH-������Һ�е���غ�Ϊc��NH4+��+c��H+��=c��Cl-��+c��OH-����
C��c�������������Һ�е���غ�Ϊc��NH4+��+c��H+��=c��Cl-��+c��OH-����
D������d������Ͱ�ˮǡ����ȫ��Ӧ��������������
B��b��pH=7����c��H+��=c��OH-������Һ�е���غ�Ϊc��NH4+��+c��H+��=c��Cl-��+c��OH-����
C��c�������������Һ�е���غ�Ϊc��NH4+��+c��H+��=c��Cl-��+c��OH-����
D������d������Ͱ�ˮǡ����ȫ��Ӧ��������������
����⣺A��a�������10mL��ˮ����Ӧ������Ϊ�Ȼ�狀Ͱ�ˮ�������Ȼ��0.01mol��һˮ�ϰ����ʵ���Ϊ0.01mol����Ӧ����Һ���Լ��ԣ�c��OH-����c��H+����c��NH
����c��Cl-������c��NH
����c��Cl-����c��OH-����c��H+������A��ȷ��
B��b��pH=7����c��H+��=c��OH-������Һ�е���غ�Ϊc��NH4+��+c��H+��=c��Cl-��+c��OH-������c��Cl-��=c��NH4+������c��NH4+��=c��Cl-����c��H-��=c��OH-������B��ȷ��
C��c�������Ѿ���������Һ��ʾ���ԣ�������Һ��һ���������غ㣺c��NH4+��+c��H+��=c��Cl-��+c��OH-������C��ȷ��
D��d��ʱ����Ͱ�ˮǡ����ȫ��Ӧ��������࣬�ټ������¶Ƚ���ֻ���Ǽ���������¶ȵ�����Һ�¶ȣ�������¶��½���ԭ��D����
��ѡ��D��
+ 4 |
+ 4 |
B��b��pH=7����c��H+��=c��OH-������Һ�е���غ�Ϊc��NH4+��+c��H+��=c��Cl-��+c��OH-������c��Cl-��=c��NH4+������c��NH4+��=c��Cl-����c��H-��=c��OH-������B��ȷ��
C��c�������Ѿ���������Һ��ʾ���ԣ�������Һ��һ���������غ㣺c��NH4+��+c��H+��=c��Cl-��+c��OH-������C��ȷ��
D��d��ʱ����Ͱ�ˮǡ����ȫ��Ӧ��������࣬�ټ������¶Ƚ���ֻ���Ǽ���������¶ȵ�����Һ�¶ȣ�������¶��½���ԭ��D����
��ѡ��D��
���������⿼��ˮ��Һ�еĵ���ƽ���Լ�����к͵ζ�����ȷ�ζ������и����pH�ǽ��Ĺؼ�����ѧ�����������غ㡢����غ���������ϰ�⣮

��ϰ��ϵ�д�
 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�
�����Ŀ
 �����£���1.00mol?L-1�������20.00mL1.00mol?L-1��ˮ�У���ҺpH�������
�����£���1.00mol?L-1�������20.00mL1.00mol?L-1��ˮ�У���ҺpH������� ��2012?������һģ�������£���1.00mol?L��1�������20.00mL 1.00mol?L��1��ˮ�У���ҺpH�������������仯����������ͼ��ʾ������˵����ȷ���ǣ�������
��2012?������һģ�������£���1.00mol?L��1�������20.00mL 1.00mol?L��1��ˮ�У���ҺpH�������������仯����������ͼ��ʾ������˵����ȷ���ǣ������� �����£���1.00mol/L�������20.00mL 1.00mol/L��ˮ�У���ҺpH���¶ȣ��棩
�����£���1.00mol/L�������20.00mL 1.00mol/L��ˮ�У���ҺpH���¶ȣ��棩