��Ŀ����
 �����£���1.00mol/L�������20.00mL 1.00mol/L��ˮ�У���ҺpH���¶ȣ��棩
�����£���1.00mol/L�������20.00mL 1.00mol/L��ˮ�У���ҺpH���¶ȣ��棩�������������仯������ͼ��ʾ��
��1�������й�˵����ȷ����
C
C
A��a����ˮ�������c��H+��=10-14mol/L
B��b�㣺c��NH
+ 4 |
C��c�㣺C��Cl-��=C��NH4+��
D��d�����Һ�¶����½�����Ҫԭ����NH3?H2O��������
��2���ڵμӹ����У�ˮ�ĵ���̶ȵı仯����
����
����
����С
��С
�����������С�����䡱������3�����±��У��ֱ�����������ʵ�����������Ũ�ȵĴ�С˳��Ӧ���ʵĻ�ѧʽ����Һ��pH������д���пհף�
| ����Ũ�ȵĹ�ϵ | ���� | ��Һ��pH | �����غ��ϵ | |
| �� | C��NH4+����C��Cl-����C��OH-����C��H+�� | NH4Cl��NH3?H2O NH4Cl��NH3?H2O |
pH��7 | / |
| �� | NH4Cl | / | c��NH4+��+c��NH3?H2O��=c��Cl-�� c��NH4+��+c��NH3?H2O��=c��Cl-�� | |
| �� | C��Cl-����C��H+����C��NH4+����C��OH-�� | NH4Cl��HCl NH4Cl��HCl |
pH��7 | / |
��������1��A��һˮ�ϰ�Ϊ������벻��ȫ������Ksp��֪ˮ������������ӣ�
B��b��Ϊһˮ�ϰ����Ȼ�淋Ļ���
C��c��pH=7����c��H+��=c��OH-������Һ�е���غ�Ϊc��NH4+��+c��H+��=c��Cl-��+c��OH-����
D��d���Ӧ�������Ũ��Խ��ԽС��
��2����ζ���Ĺ����У�����������Ũ�ȼ�С����Ӧ���յ��������Ũ������
��3����������Ũ�ȿ�֪������Ϊһˮ�ϰ����Ȼ�泥�
������ֻ���Ȼ��ʱ��笠�����ˮ�⣻
��������Ũ�ȿ�֪������ΪHCl��NH4Cl��
B��b��Ϊһˮ�ϰ����Ȼ�淋Ļ���
C��c��pH=7����c��H+��=c��OH-������Һ�е���غ�Ϊc��NH4+��+c��H+��=c��Cl-��+c��OH-����
D��d���Ӧ�������Ũ��Խ��ԽС��
��2����ζ���Ĺ����У�����������Ũ�ȼ�С����Ӧ���յ��������Ũ������
��3����������Ũ�ȿ�֪������Ϊһˮ�ϰ����Ȼ�泥�
������ֻ���Ȼ��ʱ��笠�����ˮ�⣻
��������Ũ�ȿ�֪������ΪHCl��NH4Cl��
����⣺��1��A��һˮ�ϰ�Ϊ������벻��ȫ��c��OH-����0.1mol/L������Ksp��֪ˮ������������Ӵ���c��H+��=10-13mol/L����A����
B��b��Ϊһˮ�ϰ����Ȼ�淋Ļ�����c��NH4+��+c��NH3?H2O����c��Cl-������B����
C��c��pH=7����c��H+��=c��OH-������Һ�е���غ�Ϊc��NH4+��+c��H+��=c��Cl-��+c��OH-������c��Cl-��=c��NH4+������C��ȷ��
D��d���Ӧ�������Ũ��Խ��ԽС��������Һ���¶��ڼ�С����D����
�ʴ�Ϊ��C��
��2����ζ���Ĺ����У�����������Ũ�ȼ�С����Ӧ���յ��������Ũ�������������ˮ�ĵ��룬��ˮ�ĵ���̶���������С���ʴ�Ϊ������С��
��3����������Ũ�ȿ�֪��笠�����Ũ���������Һ�Լ��ԣ�������Ϊһˮ�ϰ����Ȼ�泥��ʴ�Ϊ��NH4Cl��NH3?H2O��
������ֻ���Ȼ��ʱ��笠�����ˮ������һˮ�ϰ����������غ��֪������Ũ�ȹ�ϵΪc��NH4+��+c��NH3?H2O��=c��Cl-�����ʴ�Ϊ��c��NH4+��+c��NH3?H2O��=c��Cl-����
��������Ũ�ȿ�֪��������Ũ���������Һ�����ԣ�������ΪHCl��NH4Cl���ʴ�Ϊ��NH4Cl��HCl��
B��b��Ϊһˮ�ϰ����Ȼ�淋Ļ�����c��NH4+��+c��NH3?H2O����c��Cl-������B����
C��c��pH=7����c��H+��=c��OH-������Һ�е���غ�Ϊc��NH4+��+c��H+��=c��Cl-��+c��OH-������c��Cl-��=c��NH4+������C��ȷ��
D��d���Ӧ�������Ũ��Խ��ԽС��������Һ���¶��ڼ�С����D����
�ʴ�Ϊ��C��
��2����ζ���Ĺ����У�����������Ũ�ȼ�С����Ӧ���յ��������Ũ�������������ˮ�ĵ��룬��ˮ�ĵ���̶���������С���ʴ�Ϊ������С��
��3����������Ũ�ȿ�֪��笠�����Ũ���������Һ�Լ��ԣ�������Ϊһˮ�ϰ����Ȼ�泥��ʴ�Ϊ��NH4Cl��NH3?H2O��
������ֻ���Ȼ��ʱ��笠�����ˮ������һˮ�ϰ����������غ��֪������Ũ�ȹ�ϵΪc��NH4+��+c��NH3?H2O��=c��Cl-�����ʴ�Ϊ��c��NH4+��+c��NH3?H2O��=c��Cl-����
��������Ũ�ȿ�֪��������Ũ���������Һ�����ԣ�������ΪHCl��NH4Cl���ʴ�Ϊ��NH4Cl��HCl��
���������⿼������ϼ���Һ������Ũ�ȴ�С�Ĺ�ϵ��ע����ӦΪ���ȷ�Ӧ��ͼ���뷴Ӧ�Ķ�Ӧ��ϵ���ɽ����ȷ��Ӧ����Һ�е������ǽ����Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д� Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д�
�����Ŀ
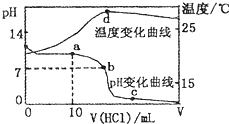 �����£���1.00mol?L-1�������20.00 mL 1.00mol?L-1�İ�ˮ�У���ҺpH���¶��������������ı仯������ͼ��ʾ�������й�˵���в���ȷ���ǣ�������
�����£���1.00mol?L-1�������20.00 mL 1.00mol?L-1�İ�ˮ�У���ҺpH���¶��������������ı仯������ͼ��ʾ�������й�˵���в���ȷ���ǣ�������A��a����Һ������Ũ�ȴ�С�Ĺ�ϵ��c��NH
| ||
B��b����Һ������Ũ�ȴ�С�Ĺ�ϵ��c��NH
| ||
C��c����Һ������Ũ�ȴ�С�Ĺ�ϵ��c��NH
| ||
| D��d��ʱ��Һ�¶ȴﵽ��ߣ�֮���¶������½���ԭ����NH3?H2O�������� |
 �����£���1.00mol?L-1�������20.00mL1.00mol?L-1��ˮ�У���ҺpH�������
�����£���1.00mol?L-1�������20.00mL1.00mol?L-1��ˮ�У���ҺpH������� ��2012?������һģ�������£���1.00mol?L��1�������20.00mL 1.00mol?L��1��ˮ�У���ҺpH�������������仯����������ͼ��ʾ������˵����ȷ���ǣ�������
��2012?������һģ�������£���1.00mol?L��1�������20.00mL 1.00mol?L��1��ˮ�У���ҺpH�������������仯����������ͼ��ʾ������˵����ȷ���ǣ�������