��Ŀ����
����Ŀ����1molN2�����3molH2������2L�ĺ��������У�����һ�������·������·�Ӧ��N2(g)+3H2(g) ![]() 2NH3(g)������2s����NH3��Ũ��Ϊ0.6mol��L-1���������м���˵�������в���ȷ����
2NH3(g)������2s����NH3��Ũ��Ϊ0.6mol��L-1���������м���˵�������в���ȷ����
A. ��N2��ʾ�ķ�Ӧ����Ϊ0.15mol��L-1��s-1 B. 2sʱH2��ת����Ϊ40��
C. 2sʱN2��H2��ת������� D. 2sʱH2��Ũ��Ϊ0.6mol��L-1
���𰸡�B
��������
����:��1molN2�����3molH2������2L�ĺ��������У�����2s����NH3��Ũ��Ϊ0.6molL-1�����ɰ���Ϊ2L��0.6mol/L=1.2mol����
N2��g��+3H2��g��2NH3��g��
��ʼ����mol����1 3 0
�仯����mol����0.61.8 1.2
2sʱ��mol����0.4 1.21.2
A.����v=![]() ������N2��ʾ�ķ�Ӧ���ʣ�
������N2��ʾ�ķ�Ӧ���ʣ�
B.����![]() =ת����/��ʼ����100%������H2��ת���ʣ�
=ת����/��ʼ����100%������H2��ת���ʣ�
C.N2��H2��ʼ���ʵ���Ϊ1��3�����߰�1��3��Ӧ����N2�� H2��ת������ȣ�
D.����c=![]() ������
������
��1molN2�����3molH2������2L�ĺ��������У�����2s����NH3��Ũ��Ϊ0.6molL-1�����ɰ���Ϊ2L��0.6mol/L=1.2mol����
N2��g��+3H2��g��2NH3��g��
��ʼ����mol����1 3 0
�仯����mol����0.6 1.8 1.2
2sʱ��mol����0.4 1.2 1.2
A.��N2��ʾ�ķ�Ӧ����Ϊ:![]() =0.15 molL-1s-1����A��ȷ��
=0.15 molL-1s-1����A��ȷ��
B. 2sʱH2��ת����Ϊ��![]() ��100%=60%;��B������
��100%=60%;��B������
C.N2��H2��ʼ���ʵ���Ϊ1��3�����߰�1��3��Ӧ����N2�� H2��ת������ȣ���C��ȷ��
D.2sʱH2��Ũ��Ϊ![]() =0.6molL-1����D��ȷ��
=0.6molL-1����D��ȷ��
���Ա����ѡB��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ����������о�����Ч���ƿ����еĵ������̼������ĺ����Ե���Ϊ��Ҫ��
I.���������о�
��1��һ�������£���2molNO��2molO2���ں����ܱ������з�����Ӧ2NO(g)+O2(g)![]() 2NO2(g)�����и�����˵����Ӧ�ﵽƽ��״̬���� ________________������ĸ���ţ� ��
2NO2(g)�����и�����˵����Ӧ�ﵽƽ��״̬���� ________________������ĸ���ţ� ��
a����ϵѹǿ���ֲ��� b�����������ɫ���ֲ���
c��NO��O2�����ʵ���֮�ȱ��ֲ��� d��ÿ����1 molO2ͬʱ����2 molNO2
��2��������ȼ������ʱ������N2��O2�ķ�Ӧ��N2 +O2![]() 2NO���ǵ�������β���к���NO��ԭ��֮һ����T1��T2�¶��£�һ������NO�����ֽⷴӦʱN2�����������ʱ��仯����ͼ��ʾ������ͼ���жϷ�ӦN2(g)+O2(g)
2NO���ǵ�������β���к���NO��ԭ��֮һ����T1��T2�¶��£�һ������NO�����ֽⷴӦʱN2�����������ʱ��仯����ͼ��ʾ������ͼ���жϷ�ӦN2(g)+O2(g)![]() 2NO(g)����H__________0(����������������)��
2NO(g)����H__________0(����������������)��

����̼�������о�
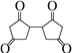
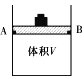
��1������ɱ䣨����������֮���Ħ�������Բ��ƣ����ܱ���������ͼ��ʾ���ֽ�3molH2��2molCO���������У��ƶ����������VΪ2L����í���̶���A��B�㣬�����ϳɼ״��ķ�Ӧ���£�CO(g)+2H2(g)![]() CH3OH(g)��
CH3OH(g)��
�ⶨ��ͬ��������ͬʱ����ڵ�CO��ת���ʣ��õ��������ݣ�
T������ | 10min | 20min | 30min | 40min |
T1 | 20% | 55% | 65% | 65% |
T2 | 35% | 50% | a1 | a2 |
�������ϱ����ݣ���Ƚ�T1_________T2(ѡ����>������<������=��)��T2���£���30min ʱ��a1=________�����¶��µĻ�ѧƽ�ⳣ��Ϊ__________________��
��T2���£���40minʱ����ȥí���������ܷ������ã�����û�з����ƶ�������������ͨ��6molCO����ʱv(����________v(�棩(ѡ����>������<����
��2��һ�������¿��ü״���CO��Ӧ���ɴ�������CO��Ⱦ�������£���a mol/L�Ĵ�����b mol/L Ba(OH)2��Һ��������(��Ϻ���Һ����仯���Բ��ƣ�����ַ�Ӧ����Һ�д���2c(Ba2+)��c(CH3COO-)���������Һ�еĵ��ƽ���֪����Һ��pH=___________��������������ĵ��볣��Ka =____________ (�ú�a��b�Ĵ���ʽ��ʾ����
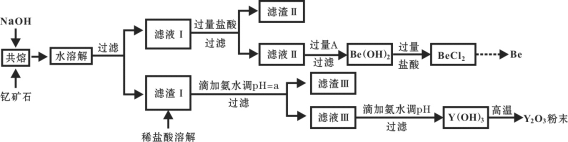
����Ŀ��������ѧ���������ϡ��������ͻ��������Ϊ��ϡ�����Ԭ¡ƽ��������ϡ��Ԫ��֮һ���ҹ��̲��ŷḻ���ƿ�ʯ(Y2FeBe2Si2O10)����ҵ��ͨ�����¹���������ȡ�����ƣ�����ø������롣
��֪�������ƣ�Y���ij������ϼ�Ϊ��3�ۣ�
�������������Ԫ�����ڱ��ĶԽ���λ�ã���ѧ�������ƣ�
����Fe3+��Y3+�γ������������ʱ��pH���±���
���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
Fe3+ | 2.1 | 3.1 |
Y3+ | 6.0 | 8.3 |
��1�����ƿ�ʯ��NaOH���۵ķ�Ӧ����ʽ����������
__Y2FeBe2Si2O10+__NaOH+____ ![]() __Y(OH)3 +__Fe2O3 + __Na2SiO3 + __Na2BeO2 + __H2O
__Y(OH)3 +__Fe2O3 + __Na2SiO3 + __Na2BeO2 + __H2O
��2�����������Ҫ�ɷ���____________��
��3���Լ�A������___________��
A��NaOH��Һ B����ˮ C��CO2 D��CaO
��4���ð�ˮ����pH=aʱ��a��ȡֵ��Χ��_____________________��
��5�����㳣����Y3+ +3H2O![]() Y(OH)3+3H+��ƽ�ⳣ��K=________����������Ksp [Y(OH)3] = 8.0��10-23��
Y(OH)3+3H+��ƽ�ⳣ��K=________����������Ksp [Y(OH)3] = 8.0��10-23��
��6����Һ����백ˮ�������������ӷ���ʽΪ_______________��
��7����BeCl2��Һ�еõ�BeCl2����IJ�����________��
��8����������BeCl2��������Be�Ĺ�ҵ���������֣��ٵ�ⷨ�����NaCl-BeCl2����������Ʊ�Be�����Ȼ�ԭ�������������£��ػ�ԭBeCl2�Ʊ�Be���������ַ�������Ϊ���ָ��ò���˵������_________��