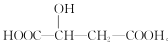��Ŀ����
����Ŀ����ˮ�������õIJ��ֹ�������ͼ��ʾ��
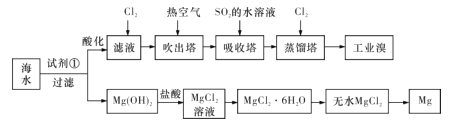
(1)������һ�������ȿ�����ˮ�������������壬����SO2��ˮ��Һ���仹ԭ���գ�������Ӧ�Ļ�ѧ����ʽΪ_____________________��
(2)Ϊ��ʹMg2+ת��ΪMg(OH)2���Լ��ٿ�����_________________(�ѧʽ)
(3)��ˮMgCl2������״̬�£�ͨ�������Mg��Cl2���÷�Ӧ�Ļ�ѧ����ʽΪ_______��
(4)�����йغ�ˮ�ۺ����õ�˵���������_____(����ĸ����)��
A.���οɲ��ó��Ӻͽᾧ�ȹ����ᴿ B.��ⱥ��ʳ��ˮ���Ƶý�����
C.����Һ��ͨ��C12��Ϊ������Br- D.��ˮ��þ�������漰���ֽⷴӦ
���𰸡�Br2 + SO2 + 2H2O =2HBr + H2SO4 Ca(OH)2  B
B
��������
(1)������Ӧ�Ļ�ѧ����ʽΪBr2 + SO2 + 2H2O =2HBr + H2SO4��
(2)Ϊ��ʹMg2+ת��ΪMg(OH)2���Լ��ٿ�����CaO�� Ca(OH)2��
(3)��Ӧ�Ļ�ѧ����ʽΪ
(4) A.�����к���Ca2+��Mg2+��SO42-�����ʣ�����ʱͨ������Һ�������м��������BaCl2��Һ��������NaOH��Һ������Na2CO3��Һ�����˺�����Һ�м�����������Һ�����ԣ��ٽ����ؽᾧ�����ᴿ����A��ȷ��
B.��ⱥ��ʳ��ˮ���ɵ����������������������ƣ���������Ȼ��������ƺ���������B����
C. ����Һ��ͨ��C12��Ϊ������Br-���������ӷ�ӦΪCl2 + 2Br- = 2Cl- + Br2�������壬��C��ȷ��
D.��ˮ��þ���̣��漰����������þ��������þ�����ᷴӦ�����Ȼ�þ����������Ȼ�þ������þ����������������þCa(OH)2+MgCl2=Mg(OH)2��+CaCl2��������þ�����ᷴӦ�����Ȼ�þMg(OH)2+2HCl= MgCl2+H2O��Ϊ���ֽⷴӦ����D��ȷ��
�ʴ�Ϊ��B��

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�����Ŀ��(8��)��¯���������з�������Ҫ��ӦΪ
![]()
��֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����£�

��ش��������⣺
(1)�÷�Ӧ��ƽ�ⳣ������ʽK=_____________����H________0(����>������<������=��)��
(2)��һ���ݻ�Ϊ10L���ܱ������У�1000��ʱ����Fe��Fe2O3��CO��CO2��1.0 mol����Ӧ����l0 min��ﵽƽ�⡣���ʱ�䷶Χ�ڷ�Ӧ��ƽ����Ӧ������(C02)= _____________��CO��ƽ��ת����= _____________��
(3)�����(2)��CO��ƽ��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_____________��
A������Fe���� | B������Fe203���� | C���Ƴ�����C02 |
D����߷�Ӧ�¶� E����С�������ݻ� F��������ʵĴ��� |
����Ŀ������˵������ȷ����
A. ��Ϊ�ʱ���ر䶼�뷴Ӧ���Է����й�,����ʱ���ر�����Ե�����Ϊ��Ӧ�Է��Ե��о�
B. 25 �桢101 kPaʱ,1 mol S��2 mol S��ȼ���Ȳ����
C. ͨ�����ǰѲ�1 molij��ѧ�������յ��������ɸû�ѧ���ļ���
��ѧ�� | H��H | Cl��Cl | H��Cl |
����1 mol��ѧ ��ʱ�ų������� | 436 kJ��mol-1 | 243 kJ��mol-1 | 431 kJ��mol-1 |
��1/2H2(g)+ 1/2Cl2(g)![]() HCl(g)����H=-183 kJ��mol-1
HCl(g)����H=-183 kJ��mol-1
D. �κλ�ѧ��Ӧ�������������ı仯