��Ŀ����
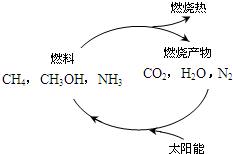
��1����Դ�ǵ�����ᷢչ������֧��֮һ����Ȼ����һ�ָ�Ч���ͺġ���ȾС�������Դ����Ҫ�ɷ�Ϊ���飬����ȼ�յĻ�ѧ����ʽΪ��
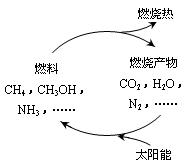
����ͼ1���������ѭ����̫��������ת��Ϊ

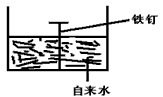
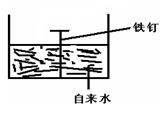
��2�������ĸ�ʴ����dz��ձ飬�绯ѧ��ʴ����ɸ�����ʴ����Ҫԭ��ijͬѧ��ͼ2���и�����ʴ��ģ�⣬���ĵ缫��ӦʽΪ
����ʾ���绯ѧ���ܷ�ӦʽΪ2Fe+2H2O+O2=2Fe��OH��2��
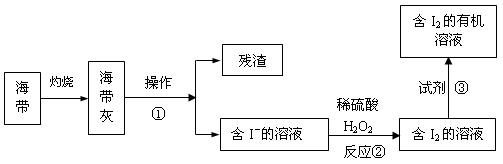
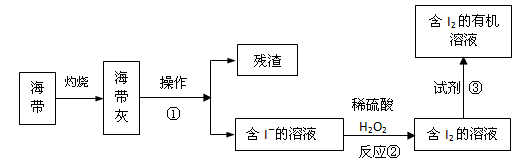
��3����ˮ��ѧ��Դ�Ŀ������þ�����Ҫ����������ã���ͼ3�Ǻ�������ȡ���ʾ��ͼ�������ٵ�������
A���Ҵ� B���� C������ D�����Ȼ�̼��
CH4+2O2
CO2+2H2O
| ��ȼ |
CH4+2O2
CO2+2H2O
����״���£�11.2L����ȼ��ʱ��ת�Ƶ��ӵ����ʵ���Ϊ| ��ȼ |
4
4
mol������ͼ1���������ѭ����̫��������ת��Ϊ
��
��
�ܣ�
��2�������ĸ�ʴ����dz��ձ飬�绯ѧ��ʴ����ɸ�����ʴ����Ҫԭ��ijͬѧ��ͼ2���и�����ʴ��ģ�⣬���ĵ缫��ӦʽΪ
2Fe-4e-=2Fe2+
2Fe-4e-=2Fe2+
�������ĵ缫��ӦʽΪO2+2H2O+4e-=4OH-
O2+2H2O+4e-=4OH-
������ʾ���绯ѧ���ܷ�ӦʽΪ2Fe+2H2O+O2=2Fe��OH��2��
��3����ˮ��ѧ��Դ�Ŀ������þ�����Ҫ����������ã���ͼ3�Ǻ�������ȡ���ʾ��ͼ�������ٵ�������
�ܽ⡢����
�ܽ⡢����
����Ӧ�ڵ����ӷ���ʽ��H2O2+2I-+2H+=2H2O+I2
H2O2+2I-+2H+=2H2O+I2
�����������в������Լ��۵���AC
AC
��A���Ҵ� B���� C������ D�����Ȼ�̼��
��������1������ȼ�����ɶ�����̼��ˮ�����ݼ����ת�Ƶ���֮��Ĺ�ϵʽ���㣬̫��������ת��Ϊ��ѧ�ܣ���λ�ѧ��ת��Ϊ���ܣ�
��2����װ������ԭ��أ�����������̼��������������ʧ���ӷ���������Ӧ�������ϵõ��ӷ�����ԭ��Ӧ��
��3�����벻���Թ������Һ�ķ������ܽ⡢���ˣ������Ӻ�˫��ˮ����������ԭ��Ӧ���ɵⵥ�ʺ�ˮ��������ȡ����ѡȡ���жϣ�
��2����װ������ԭ��أ�����������̼��������������ʧ���ӷ���������Ӧ�������ϵõ��ӷ�����ԭ��Ӧ��
��3�����벻���Թ������Һ�ķ������ܽ⡢���ˣ������Ӻ�˫��ˮ����������ԭ��Ӧ���ɵⵥ�ʺ�ˮ��������ȡ����ѡȡ���жϣ�
����⣺��1������ȼ�����ɶ�����̼��ˮ����Ӧ����ʽΪ��CH4+2O2
CO2+2H2O�����ݷ���ʽ֪����״���£�11.2L����ȼ��ʱ��ת�Ƶ��ӵ����ʵ���=
��8=4mol��
̫��������ת��Ϊ��ѧ�ܣ���λ�ѧ��ת��Ϊ���ܣ�
�ʴ�Ϊ��CH4+2O2
CO2+2H2O��4���ȣ�
��2����װ������ԭ��أ�����������̼��������������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��2Fe-4e-=2Fe2+�������ϵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ��O2+2H2O+4e-=4OH-��
�ʴ�Ϊ��2Fe-4e-=2Fe2+��O2+2H2O+4e-=4OH-��
��3�����벻���Թ������Һ�ķ������ܽ⡢���ˣ������Ӻ�˫��ˮ����������ԭ��Ӧ���ɵⵥ�ʺ�ˮ�����ӷ���ʽΪ��H2O2+2I-+2H+=2H2O+I2����ȡ����ѡȡ��Ϊ����������ȡ���е��ܽ�ȴ�����ԭ�ܼ��е��ܽ�ȣ������ܼ����ܻ��ܣ���ȡ�������ʲ���Ӧ���Ҵ�����������ˮ���ܣ����Բ�������ȡ�����������Ȼ�̼������ȡ����ѡȡ�������Կ�������ȡ������ѡAC��
�ʴ�Ϊ���ܽ⡢���ˣ�H2O2+2I-+2H+=2H2O+I2��AC��
| ��ȼ |
| 11.2L |
| 22.4L/mol |
̫��������ת��Ϊ��ѧ�ܣ���λ�ѧ��ת��Ϊ���ܣ�
�ʴ�Ϊ��CH4+2O2
| ��ȼ |
��2����װ������ԭ��أ�����������̼��������������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��2Fe-4e-=2Fe2+�������ϵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ��O2+2H2O+4e-=4OH-��
�ʴ�Ϊ��2Fe-4e-=2Fe2+��O2+2H2O+4e-=4OH-��
��3�����벻���Թ������Һ�ķ������ܽ⡢���ˣ������Ӻ�˫��ˮ����������ԭ��Ӧ���ɵⵥ�ʺ�ˮ�����ӷ���ʽΪ��H2O2+2I-+2H+=2H2O+I2����ȡ����ѡȡ��Ϊ����������ȡ���е��ܽ�ȴ�����ԭ�ܼ��е��ܽ�ȣ������ܼ����ܻ��ܣ���ȡ�������ʲ���Ӧ���Ҵ�����������ˮ���ܣ����Բ�������ȡ�����������Ȼ�̼������ȡ����ѡȡ�������Կ�������ȡ������ѡAC��
�ʴ�Ϊ���ܽ⡢���ˣ�H2O2+2I-+2H+=2H2O+I2��AC��
���������⿼����ۺϣ��漰ԭ���ԭ������ȡ����ѡȡ��֪ʶ�㣬�ѶȲ���ע����ȡ����ѡȡ������Ϊ������㣮

��ϰ��ϵ�д�
 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����Ŀ
 ��Դ�ǵ�����ᷢչ������֧��֮һ��
��Դ�ǵ�����ᷢչ������֧��֮һ��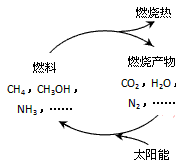
 ����ӦʽΪ ��
����ӦʽΪ ��