��Ŀ����
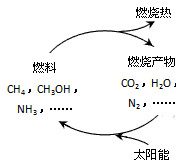
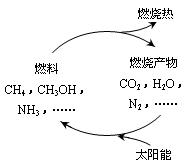
��1����Դ�ǵ�����ᷢչ������֧��֮һ����Ȼ����һ�ָ�Ч���ͺġ���ȾС�������Դ����Ҫ�ɷ�Ϊ���飬����ȼ�յĻ�ѧ����ʽΪ�� ����״���£�11.2L����ȼ��ʱ��ת�Ƶ��ӵ����ʵ���Ϊ mol��s
����ͼ���������ѭ����̫��������ת��Ϊ �ܡ�
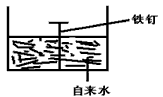
��2�������ĸ�ʴ����dz��ձ飬�绯ѧ��ʴ����ɸ�����ʴ����Ҫԭ��ijͬѧ����ͼ���и�����ʴ��ģ�⣬��
�����ĵ� ����ӦʽΪ ��
����ӦʽΪ ��
�����ĵ缫��ӦʽΪ ��
����ʾ���绯ѧ���ܷ�ӦʽΪ2Fe+2H2O+O2=2Fe(OH)2��
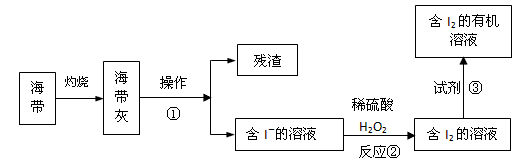
��3����ˮ��ѧ��Դ�Ŀ������þ�����Ҫ����������ã���ͼ�Ǻ�������ȡ���ʾ��ͼ��
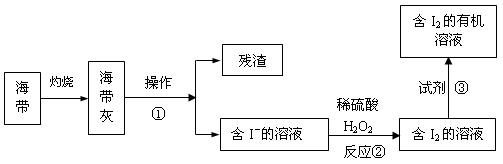
�����ٵ������� ����Ӧ�ڵ����ӷ���ʽ�� �����������в������Լ��۵��� ��
A���Ҵ� B���� C������ D�����Ȼ�̼
����14�֣���1��![]() ��2�֣� 4�� �ȡ�
��2�֣� 4�� �ȡ�
��2��2Fe �� 4e- = 2Fe2+����2�֣� O2 + 2H2O + 4e- = 4OH-��2�֣�
��3���ܽ⡢���ˣ���2�֣� H2O2 +2I-+2H+ = 2H2O + I2�� ��2�֣� AC����2�֣�

 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д�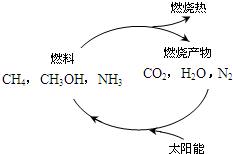 ��Դ�ǵ�����ᷢչ������֧��֮һ��
��Դ�ǵ�����ᷢչ������֧��֮һ��