��Ŀ����
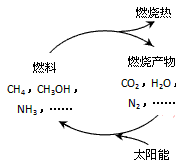
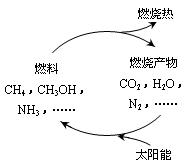
��1����Դ�ǵ�����ᷢչ������֧��֮һ����Ȼ����һ�ָ�Ч���ͺġ���ȾС�������Դ����Ҫ�ɷ�Ϊ���飬����ȼ�յĻ�ѧ����ʽΪ��__________ �� ��״���£�11.2L����ȼ��ʱ��ת�Ƶ��ӵ����ʵ���Ϊ__________ mol�� ������ͼ���������ѭ����̫��������ת��Ϊ _________�ܡ�
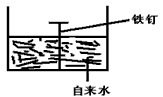
��2�������ĸ�ʴ����dz��ձ飬�绯ѧ��ʴ����ɸ�����ʴ����Ҫԭ��ijͬѧ����ͼ���и�����ʴ��ģ�⣬���ĵ缫��ӦʽΪ________ �� �����ĵ缫��ӦʽΪ _________�� ����ʾ���绯ѧ���ܷ�ӦʽΪ2Fe+2H2O+O2==2Fe(OH)2��
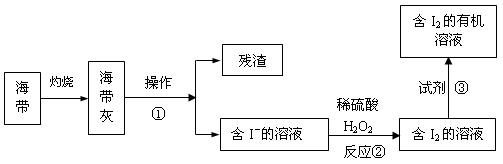
��3����ˮ��ѧ��Դ�Ŀ������þ�����Ҫ����������ã���ͼ�Ǻ�������ȡ���ʾ��ͼ��
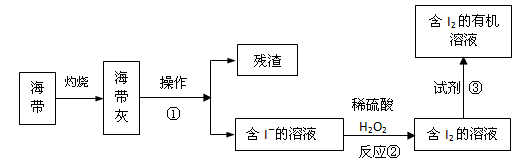
�����ٵ�������_________ ����Ӧ�ڵ����ӷ���ʽ��__________ �����������в������Լ��۵���____________ ��
A���Ҵ�
B����
C������
D�����Ȼ�̼
A���Ҵ�
B����
C������
D�����Ȼ�̼
��1��CH4 +2O2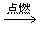 CO2 +2H2O��4�� �ȡ�
CO2 +2H2O��4�� �ȡ�
��2��2Fe - 4e- == 2Fe2+�� O2 + 2H2O + 4e- = 4OH-
��3���ܽ⡢���ˣ� H2O2 +2I-+2H+ = 2H2O + I2�� AC��
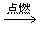 CO2 +2H2O��4�� �ȡ�
CO2 +2H2O��4�� �ȡ� ��2��2Fe - 4e- == 2Fe2+�� O2 + 2H2O + 4e- = 4OH-
��3���ܽ⡢���ˣ� H2O2 +2I-+2H+ = 2H2O + I2�� AC��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
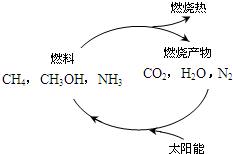 ��Դ�ǵ�����ᷢչ������֧��֮һ��
��Դ�ǵ�����ᷢչ������֧��֮һ��
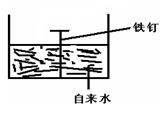
 ����ӦʽΪ ��
����ӦʽΪ ��