��Ŀ����
����Ŀ���ú�������ͭ��[��CuO��Cu2(OH)2CO3��As2O3���ؽ����ε�]��ȡCu2(OH)2SO4�Ĺ����������£�
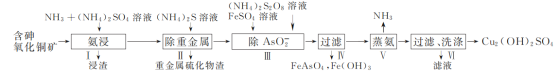
(1) �������������ʱ�������¶�Ϊ50��55 �棬pHԼΪ9.5����ͭ������ת��Ϊ[Cu(NH3)4]SO4��Һ��
�� CuO����ȡ�����ӷ���ʽΪ________��
�ڽ�ȡ�¶Ȳ��˳���55 �棬��ԭ����________��
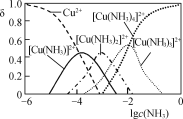
�� Cu2����NH3���ʱ����Һ�к�ͭ�������ʵ����ֲ�����(��)����Һ�������c(NH3)�Ķ���ֵ�Ĺ�ϵ��ͼ��ʾ������1 L��ȡҺ(�ɵ����ʵ���NH3��NH4+���)��amolCu2(OH)2CO3ȫ������Ϊ[Cu(NH3)4]2��(CO32-ת��ΪHCO3-��������������Ӧ����Һ����仯���Բ���)��ԭ��ȡҺ����ʼʱc(NH3)Ӧ�����������________��
(2) ����AsO2-��ʱ��FeSO4�������һ����ʹAsO2-������ȫ����һĿ����________��
(3) ��������ʱ������Ӧ�Ļ�ѧ����ʽΪ________��
(4) Ϊ��ʵ����������������ѭ�����ã��ɲ�ȡ�Ĵ�ʩΪ________��
���𰸡�CuO+2NH3+2NH4+=[Cu(NH3)4]2��+H2O �¶ȹ��ߣ������NH3�Ļӷ� c(NH3)��(5a��1) mol��L��1 ���ɵ�Fe(OH)3[��Fe(OH)3��Fe(OH)2]��״��������FeAsO4�Ⱥ����� 2[Cu(NH3)4]SO4![]() Cu2(OH)2SO4����6NH3��+(NH4)2SO4 �ò����������NH3�벽�������Һ���Ʋ���������NH3��(NH4)2SO4��ȡҺ
Cu2(OH)2SO4����6NH3��+(NH4)2SO4 �ò����������NH3�벽�������Һ���Ʋ���������NH3��(NH4)2SO4��ȡҺ
��������
�������̣���NH3��(NH4)2SO4��ɵ���Һ������������ͭ��[��CuO��Cu2(OH)2CO3��As2O3���ؽ����ε�]����ͭ������ת��Ϊ[Cu(NH3)4]SO4��Һ��ͬʱ�����ؽ����κ�AsO2-��������麟�ȥ�ؽ������ӣ��ټ���(NH4)2S2O8��FeSO4������AsO2-������FeAsO4��Fe(OH)3�������õ�����Һ��ҪΪ(NH4)2SO4��[Cu(NH3)4]SO4��Һ�����������ˡ�ϴ�ӵõ���ƷCu2(OH)2SO4��
(1)��CuO��NH3��(NH4)2SO4��ɵ���Һ��ȡ�õ�[Cu(NH3)4]SO4���ݴ���д��
���¶ȸߣ�����ٰ����Ļӷ���
����ͼ��֪��ȫ������Ϊ[Cu(NH3)4]2+ʱlgc(NH3)=0�����ʱ����Ũ��c(NH3)=1mol/L���ٽ�Ϸ�Ӧ��Cu2(OH)2CO3+5NH3+3NH4+�T2[Cu(NH3)4]2++HCO3-+2H2O��������ɵã�
(2)���������еõ�FeAsO4��Fe(OH)3����������
(3)��������ʱ������ӦΪ[Cu(NH3)4]SO4�ֽ�Cu2(OH)2SO4�Ͱ��������ԭ���غ�ɵã�
(4)�������̷����ɵõ�NH3-(NH4)2SO4��ȡҺѭ��ʹ�á�
(1)CuO��NH3��(NH4)2SO4��ɵ���Һ��ȡ�õ�[Cu(NH3)4]SO4����Ӧ�����ӷ�ӦΪ��CuO+2NH3+2NH4+=[Cu(NH3)4]2++H2O��
�ڽ�ȡ�¶Ȳ��˳���55�棬���Ƿ�Ӧ�¶ȹ��ߣ������NH3�Ļӷ���
��a molCu2(OH)2CO3ȫ������Ϊ[Cu(NH3)4]2+�ķ�ӦΪ��Cu2(OH)2CO3+5NH3+3NH4+=2[Cu(NH3)4]2++HCO3-+2H2O����������뷴Ӧ�İ���Ϊ5amol��Ũ��Ϊ5amol/L����ͼ��֪��ȫ������Ϊ[Cu(NH3)4]2+ʱ��lgc(NH3)=0�����ʱ����Ũ��c(NH3)=1mol/L�����ԭ��ȡҺ����ʼʱc(NH3)��(5a+1)mol/L��
(2)�����У�����AsO2-��ʱ�õ�FeAsO4��Fe(OH)3������FeSO4�������һ����ʹAsO2-������ȫ����һĿ�������ɵ�Fe(OH)3[��Fe(OH)3-Fe(OH)2]��״��������FeAsO4�Ⱥ�������
(3)�������̣���������ʱ������ӦΪ[Cu(NH3)4]SO4�ֽ�Cu2(OH)2SO4�Ͱ�������Ӧ����ʽΪ��
2[Cu(NH3)4]SO4![]() Cu2(OH)2SO4����6NH3����(NH4)2SO4��
Cu2(OH)2SO4����6NH3����(NH4)2SO4��
(4)�ò����������NH3�벽�������Һ���Ʋ���������NH3-(NH4)2SO4��ȡҺ��������������������ѭ�����á�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�