��Ŀ����
����Ŀ�������й�ʵ������������ͻ���۶���ȷ����
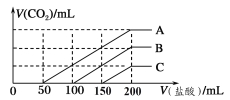
ѡ�� | ʵ����� | ���� | ���ͻ���� |
A | ���������Na2SiO3 ������Һ��Сľ�������ɺ���ھƾ���������� | Сľ����ȼ�� | Na2SiO3 ��������� |
B | �� H2 �ڳ���Cl2 �ļ���ƿ��ȼ�� | ����ƿ���Ϸ��а������� | H2��Cl2 ��������HCl |
C | �� SO2 ͨ�����Ը��������Һ�� | ��Һ��ɫ | SO2 ���������� |
D | ��ȥ��������Ĥ���������ھƾ����ϳ�ּ��� | �����ܵ������� | ���۵�ߣ�û���ۻ� |
A.AB.BC.CD.D
���𰸡�A
��������
A�����������![]() ������Һ��Сľ�������ɺ���ھƾ���������ȣ�Сľ����ȼ�գ�֤��
������Һ��Сľ�������ɺ���ھƾ���������ȣ�Сľ����ȼ�գ�֤��![]() ��ȼ�գ��������������A ��ȷ��
��ȼ�գ��������������A ��ȷ��
B�����ڳ���![]() �ļ���ƿ��ȼ�������Ȼ��⣬�Ȼ��⼫������ˮ���ڼ���ƿ���Ϸ��γ�����СҺ�Σ����а������ɣ���B����
�ļ���ƿ��ȼ�������Ȼ��⣬�Ȼ��⼫������ˮ���ڼ���ƿ���Ϸ��γ�����СҺ�Σ����а������ɣ���B����
C����![]() ͨ�����Ը��������Һ�У���Һ��ɫ�����������������������ǻ�ԭ�ԣ�����Ư���ԣ���C����
ͨ�����Ը��������Һ�У���Һ��ɫ�����������������������ǻ�ԭ�ԣ�����Ư���ԣ���C����
D������A��������Ӧ�������������۵�ߣ��������ھƾ��ƻ����ϼ����ۻ��������䣬���������۵�ߣ���D����
��ѡA��

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�����Ŀ����CO2Ӧ�����������ȼ�ϼ״������ܻ�������ЧӦ��Ӱ�죬����Ϊ��Դ���Ʊ������µ���������ϳɷ�ӦΪCO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)���ش��������⣺
CH3OH(g)+H2O(g)���ش��������⣺
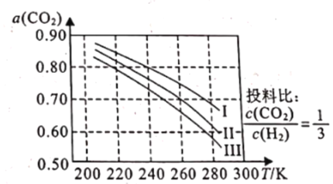
��1����ͼΪCO2ƽ��ת���ʺ��¶ȡ�ѹǿ�Ĺ�ϵ������ѹǿ�ֱ�Ϊ3.0MPa��4.0MPa��5.0MPa����ͼ��֪���÷�ӦΪ_______________��Ӧ������ȡ�������")����CO2�ij�ʼŨ��ΪcomolL-1������5.0MPaʱ�����ݼ���÷�Ӧ��ƽ�ⳣ��K(240k)=_______________ (�г�����ʽ���ɣ�������4.0MPaʱ��СͶ�ϱȣ���CO2��ƽ��ת�������߿���λ��II�ߵ�_______________����Ϸ������·���)��
��2�����ö�����̼�Ƶõļ״���������ȡ�װ����䷴Ӧԭ��ΪCH3OH(g)+NH3(g)![]() CH3NH2(g)+H2O(g)��H����֪�÷�Ӧ����ػ�ѧ���ļ����������£�
CH3NH2(g)+H2O(g)��H����֪�÷�Ӧ����ػ�ѧ���ļ����������£�
���ۼ� | C��O | H��O | N��H | C��N |
����/k.Jmol-1 | 351 | 463 | 393 | 293 |
��÷�Ӧ�ġ�H=_______________k.Jmol-1 ��
��3����֪����CO(g)+NO2(g)![]() CO2(g)+NO(g) ��H1=-226kJrnol-1
CO2(g)+NO(g) ��H1=-226kJrnol-1
��N2(g)+2O2(g)![]() 2NO2(g)��H2=+68kJmol-1
2NO2(g)��H2=+68kJmol-1
��N2(g)+O2(g)![]() 2NO(g) ��H3=+183kJmol-1
2NO(g) ��H3=+183kJmol-1
��2CO(g)+2NO(g)![]() 2CO2(g)+N2(g) ��H=_______________kJmol-1��
2CO2(g)+N2(g) ��H=_______________kJmol-1��
��4��һ���¶��£����д�ʩһ���ܼӿ췴ӦCO2(g)+3H2��g)![]() CH3OH(g)+H2O(g)�����ʵ���_______________����ѡ����ĸ����
CH3OH(g)+H2O(g)�����ʵ���_______________����ѡ����ĸ����
A.��ʱ��ȥ�״� B.�Ľ����� C.��߷�Ӧ��Ũ�� D.��������ѹǿ
��5������������ѡ������NiO- Al2O3��Ϊ��������ҵ�ϳ���Ni(NO3)2��Al(NO3)3���Һ���백ˮ����pH=12(���£���Ȼ����Һ��ѹ���·��ü����յȲ����Ʊ��ô��������백ˮ����pH=12ʱ��c(Ni2+)Ϊ_______________������֪��Ksp[Ni(OH)2]=5��10-16]