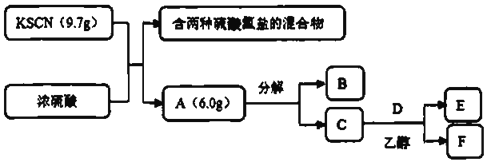
��Ŀ����
����Ŀ��������������(FeSO4��7H2O)��ҽҩ������Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£���ش��������⣺
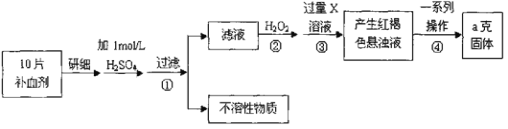
(1)ʵ��������ҩƬ��Ҫ��������_____________��
A���ձ� B���в� C������
(2)֤���������Һ�к���Fe2+�ķ������ȵμ�KSCN��Һ���ٵμ�____________���ù��̵�����Ϊ��__________________________________________________________��
(3)����ڼ������H2O2��Ŀ�ģ�________________________________________��
(4)����ڢ��з�Ӧ�����ӷ���ʽ����________________����_________________��
(5)�������һϵ�д����IJ������裺���ˡ�___�����ա�____��������
(6)��ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����_____________g��
���𰸡�B ��ˮ��˫��ˮ ��Һ��dz��ɫ��ΪѪ��ɫ ��������������Ϊ���������� H2O2+2Fe2++2H+��2Fe3++2H2O Fe3+��3OH��===Fe(OH)3�� ��Fe3+ + 3NH3��H2O =Fe(OH)3��+ 3NH4+ ϴ�� ��ȴ ![]()
��������
������ͼ��֪����ʵ��ԭ��Ϊ����ԼƷ�е�![]() �γ���Һ����
�γ���Һ����![]() ����Ϊ
����Ϊ![]() ��ʹ
��ʹ![]() ת��Ϊ����������������ת��Ϊ��������ͨ���ⶨ�����������������㲹Ѫ������Ԫ�صĺ��� (1).����ҩƬ��������Ϊ�в���
ת��Ϊ����������������ת��Ϊ��������ͨ���ⶨ�����������������㲹Ѫ������Ԫ�صĺ��� (1).����ҩƬ��������Ϊ�в���
��2���������ӵ���Һ�еμ�![]() ��Һ����Ϊ��ɫ�������������Ӻ������ӵ����ʲ������ش�
��Һ����Ϊ��ɫ�������������Ӻ������ӵ����ʲ������ش�
��3��˫��ˮ���������ԣ������������ܽ� ![]() ȫ������Ϊ
ȫ������Ϊ![]() ��������ͼ��֪������
��������ͼ��֪������![]() �ǽ�
�ǽ�![]() ����Ϊ
����Ϊ![]() ��
��
��4�� �������![]() ��
��![]() ת��Ϊ
ת��Ϊ![]() �Ĺ��̣�����
�Ĺ��̣�����![]() ���
���![]() �Ĺ��̣�
�Ĺ��̣�
��5��������ǽ�![]() ת��Ϊ���������������������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ�ӵ�����������Ȼ��������������������ȴ�������������������
ת��Ϊ���������������������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ�ӵ�����������Ȼ��������������������ȴ�������������������
��6��������Ԫ���غ��֪ag����������Ԫ�ص�������Ϊ10Ƭ��Ѫ���������������ݴ� ���㣻
(1) ����ҩƬ��������Ϊ�в���
(2)�������ӵ���Һ�еμ�![]() ��Һ����Ϊ��ɫ����ʵ��������ȵμ�
��Һ����Ϊ��ɫ����ʵ��������ȵμ�![]() ����Һ�ޱ仯���ٵμ���ˮ��˫��ˮ����������������Һ��dz��ɫ���Ѫ��ɫ��
����Һ�ޱ仯���ٵμ���ˮ��˫��ˮ����������������Һ��dz��ɫ���Ѫ��ɫ��
(3)˫��ˮ���������ԣ������������ܽ� ![]() ȫ������Ϊ
ȫ������Ϊ![]() ��������ͼ��֪������
��������ͼ��֪������![]() �ǽ�/span>
�ǽ�/span>![]() ����Ϊ
����Ϊ![]() ��
��
(4) ����![]() ��
��![]() ת��Ϊ
ת��Ϊ![]() �Ĺ��̣������ӷ���ʽΪ
�Ĺ��̣������ӷ���ʽΪ![]() ������
������![]() ���
���![]() �Ĺ��̣���
�Ĺ��̣���![]() ��
��
(5)������ǽ�![]() ת��Ϊ���������������������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ�ӵ�����������Ȼ��������������������ȴ��������������������ʴ�Ϊϴ�ӣ���ȴ��
ת��Ϊ���������������������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ�ӵ�����������Ȼ��������������������ȴ��������������������ʴ�Ϊϴ�ӣ���ȴ��
(5)������Ԫ���غ��֪ag����������Ԫ�ص�������Ϊ10Ƭ��Ѫ������������������ÿƬ��Ѫ������Ԫ�غ���Ϊ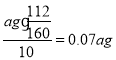 ��
��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�����Ŀ�������й�ʵ������������ͻ���۶���ȷ����
ѡ�� | ʵ����� | ���� | ���ͻ���� |
A | ���������Na2SiO3 ������Һ��Сľ�������ɺ���ھƾ���������� | Сľ����ȼ�� | Na2SiO3 ��������� |
B | �� H2 �ڳ���Cl2 �ļ���ƿ��ȼ�� | ����ƿ���Ϸ��а������� | H2��Cl2 ��������HCl |
C | �� SO2 ͨ�����Ը��������Һ�� | ��Һ��ɫ | SO2 ���������� |
D | ��ȥ��������Ĥ���������ھƾ����ϳ�ּ��� | �����ܵ������� | ���۵�ߣ�û���ۻ� |
A.AB.BC.CD.D
����Ŀ�������ж����Ҵ�����ȡ���ѶȽϴ���Ⱦ���أ������������о����������ʿ�ʵ������ɫʵ��������������ĵ�ΰ������Ѩ�еμ��������������Լ�(��Ư��������)��Ȼ��ֱ�μ���Ҫ����ʵ����Լ�����ͼ��������ijѧ���۲쵽������������ۣ�������������ʵ����
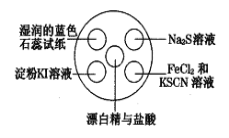
ѡ�� | ʵ������ | ���� | ���� |
A | ����KI��Һ���� | �����û���KI�еĵ�ʹ���۱��� | ���������ԣ�Cl2��I2 |
B | Na2S��Һ�г��ֵ���ɫ���� | �����û���Na2S�е��� | ���������ԣ�Cl2��S |
C | ʪ�����ɫʯ����ֽ�ȱ�����ɫ | ������ˮ��Ӧ��������ʹ����� | ��������Ư���� |
D | FeCl2��KSCN��Һ��СҺ�α�� | FeCl2��Cl2������FeCl3������KSCN��Һ��Ӧ��� | �������������� |
A.AB.BC.CD.D