��Ŀ����
����Ŀ���������ڵ�Ԫ�أ��磺��(22Ti)����(26Fe)���顢����п�ȼ�����ػ������ڻ�����ҽҩ�����ϵ��������Ź㷺��Ӧ�á��ش��������⣺
(1)��̬Tiԭ���У�����ܲ���ӵĵ�����������״Ϊ___________����Tiͬ���ڵ����й���Ԫ�صĻ�̬ԭ���У��������������Ѳ�ͬ��Ԫ����______�֡�
(2)����������Ƭ������ȱ����ƶѪ��Ԥ�������Ƶij���ҩ��ٴ��������ά����C�ٽ��������������գ���������Fe3+���ӽṹ�Ƕ�������Fe2+ �ױ�������Fe3+��ԭ����______________��
(3)SCN-���ӿ�����Fe3+�ļ��飬���Ӧ���������֣��ֱ�Ϊ������(H-S-C��N)����������(H-N=C=S)��
��д����SCN-��Ϊ�ȵ������һ����_________________(���ӻ�����)��
������������Цм��ͦҼ��ĸ���֮��Ϊ___________��
����������ķе��������е�ߵ�ԭ����________________________��
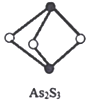
(4)����ſڴƻơ��еĴƻƷ���ʽΪAs2S3�����ӽṹ��ͼ��Asԭ�ӵ��ӻ���ʽΪ____________���ƻƺ�SnCl2�������з�Ӧת��Ϊ�ƻƣ�As4S4����SnCl4���ų�H2S���壬д���÷�Ӧ����ʽ__________________________��SnCl4���ӵĿռ乹��Ϊ______________��
(5)���ӻ�����CaC2��һ�־���ṹ��ͼ��ʾ�������ʵĵ���ʽ___________��һ���������еĦм�ƽ����___________����
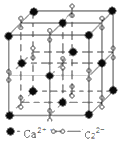
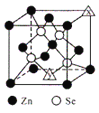
(6)����п�ľ����ṹ��ͼ��ʾ��ͼ��X��Y�����ѻ���ԭ�Ӿ�Ϊ___________(��Ԫ�ط���)���þ�������ԭ��������϶����Ϊ___________(������塱�����������塱���������塱)�����ֿ�϶�������Ϊ___________�����þ����ܶ�Ϊpgcm-3������п��Ħ������ΪMgmol-1����NA���������ӵ���������ֵ��������a Ϊ___________nm��
���𰸡� ���� 2 Fe3+��3d5����״̬���ȶ� N2O(��CO2��CS2��OCN-) 2��3 ����������Ӽ京����� sp3�ӻ� 2As2S3+2SnCl2+4HCl=As4S4+2SnCl4+2H4S�� ���������� ![]() 8 Zn �������� 50%
8 Zn �������� 50% ![]() ��107
��107
��������(1)��̬Tiԭ�ӵļ۲�����Ų�ʽΪ��3d24s2������ܲ�Ϊ�����ܲ㣬s������������״Ϊ���Σ���Tiͬ���ڵ����й���Ԫ�صĻ�̬ԭ���У��������������Ѳ�ͬ��Ԫ����Cr 3d54s1��Cu 3d104s1�������֣�
��2���ӽṹ�Ƕ�������Fe2+ �ļ۵����Ų�ʽ�ǣ�3d6����ʧһ�����Ӿ���3d5������ȶ��ṹ�����ױ�������Fe3+��
(3) ����SCN-��Ϊ�ȵ������һ�����ǣ�N2O(��CO2��CS2��OCN-)��
��������(H-S-C��N)�Цм��ͦҼ��ĸ���֮��Ϊ2��3��
������������Ӽ�����γ����������е��������е�ߣ�
(4) �ƻƷ���ʽΪAs2S3��As���ĶԼ۲���Ӷԣ�Asԭ�ӵ��ӻ���ʽΪsp3�ӻ���
�ƻƺ�SnCl2�������з�Ӧת��Ϊ�ƻƣ�As4S4����SnCl4���ų�H2S���壬���䷴Ӧ����ʽΪ��2As2S3+2SnCl2+4HCl=As4S4+2SnCl4+2H4S����SnCl4���ӵĿռ乹��Ϊ�������壻
(5)CaC2Ϊ���ӻ��������ʽΪ��![]() ��һ����������4��Ca2+��4��C22-,һ��C22-���������м���һ���������еĦм�ƽ����8����
��һ����������4��Ca2+��4��C22-,һ��C22-���������м���һ���������еĦм�ƽ����8����
(6) ����п�ľ����ṹ��X��Y�����ѻ���ԭ�Ӿ�ΪZn���þ������ĸ���ԭ������������Խ����ķ�֮һ������϶����Ϊ�������壬��ֻռ��������4��λ�ã��ʸ��ֿ�϶�������Ϊ50%�����þ����ܶ�Ϊpgcm-3������п��Ħ������ΪMgmol-1����NA���������ӵ���������ֵ��������a Ϊ![]() ��107nm��
��107nm��

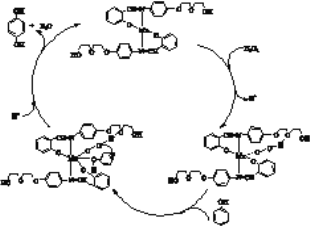
����Ŀ�����Ų��Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ��,������Ϊ���Ͻ�ά���ء�����ҵ�ϻ��շϷ�����������V2O5��VOSO4�������Բ�������������Ա����������һ�����ӽ��������շ����¹��գ������ʴ�91��7%���ϡ��ù��յ���Ҫ������ͼ��ʾ��
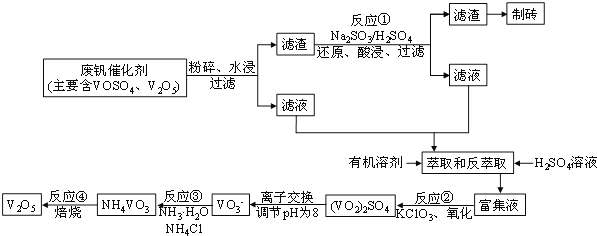
��֪���ֺ���������ˮ�е��ܽ������±���ʾ��
���� | VOSO4 | V2O5 | NH4VO3 | ��VO2��2SO4 |
�ܽ��� | ���� | ���� | ���� | ���� |
���ʴ��������⣺
��1����ҵ����V2O5ұ���������������ȼ������仯ѧ����ʽ�ɱ�ʾΪ____��
��2����Һ�к�������Ҫ�ɷ�________��д��ѧʽ������Ӧ�ٵ����ӷ���ʽ___________________��
��3����ȡ�ͷ���ȡ�������������Ҫ��������Ϊ_____________��������ȡʹ��������������һ������������_______���ѧʽ������������ɳɱ�����
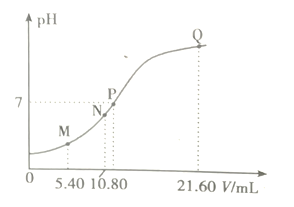
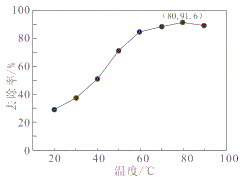
��4���ù��շ�Ӧ�۵ij����ʣ��ֳƳ����ʣ��ǻ��շ��Ĺؼ�֮һ��д���ò�������Ӧ�����ӷ���ʽ___________________���������������У����������¶ȡ��Ȼ��ϵ����NH4Cl�����������pH֮�����Һ��VO3-�������ȣ��ȵ�Ӱ�죬�����¶�������ʵĹ�ϵ��ͼ��ʾ���¶ȸ���80������ʽ��͵Ŀ���ԭ����___________________________��

��5��25��ʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���±���
pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
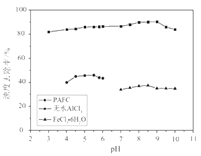
����ϱ�����ʵ�������У����м��백ˮ��������Һ�����pHֵΪ____������������Ϊ93.1%ʱ������Fe��OH��3����������Һ��c��Fe3+����_____������֪��25��ʱ��Ksp[Fe��OH��3]��2.6��10-39��
��6���Ϸ�������V2O5����������Ϊ6%��ԭ���е����з��ѻ����V2O5����ȡ100g�˷Ϸ��������������̽���ʵ�飬������105mL��0.1molL-1��KClO3��Һʱ����Һ�еķ�ǡ�ñ���ȫ�����������Ժ������û����ʧ����ù�ҵ�����з��Ļ�������______________��
����Ŀ�������ڹ�ũҵ���������Ƽ���������ҪӦ�ã����й����߶�������߹㷺�о����ش�
(1)ij������ʵ�����ڳ�ʪ��ѹ��,�Ե�����Һ̬ˮΪԭ���Ʊ�����ͬʱ���������ɡ�
��֪����һ���¶Ⱥ�ѹǿ�£������ȶ��ĵ�������1mol�����ʵ���ЧӦ����Ϊ�����ʵ�������(��H)�����³�ѹ�¡�������ʵ����������±���ʾ:
���� | NH3(s) | H20(1) |
��H/ kJ��mo1-1 | -46 | -242 |
�����ϳɰ���Ӧ���Ȼ�ѧ����ʽΪ______________________��
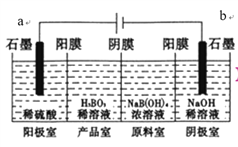
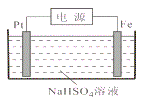
(2)���������أ���H2��N2Ϊԭ�Ϻϳɰ���װ������ͼ��ʾ��

Q��R��Ϊ�������ݵ�ʾ�жϣ�������Ӧ�Ĵ���Ϊ___(�Q����R��)�������ĵ缫��ӦʽΪ_______________��
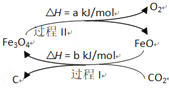
(3)�����ǹ�ҵ���������Ҫԭ��֮һ�������������з�������Ҫ��Ӧ���£�
I.4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g)��H=-906kJ/mol
4NO(g)+6H2O(g)��H=-906kJ/mol
II.4NH3(g)+3O2(g)![]() 2N2(g)+ 6H2O(g)��H=-126kJ/mol
2N2(g)+ 6H2O(g)��H=-126kJ/mol
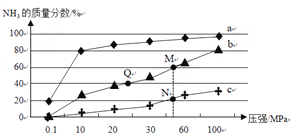
���̶�����NH3��O2�Ļ��������һ������ͨ������д����ķ�Ӧ������Ӧ�������¶ȵĹ�ϵ��ͼ1��ʾ��
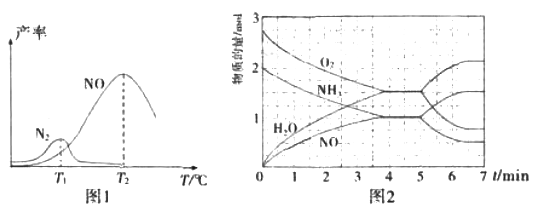
�ٴ����������У������˵��¶�Ϊ____(�T1����T2��)��
�ڵ���T1��ʱ��NO�IJ��ʽϵ͵�ԭ��Ϊ_____��
�۸���T2��ʱ��NO�IJ��ʽ��͵Ŀ���ԭ��Ϊ_____(��ѡ����ĸ)
A.�������Խ��� B.ƽ�ⳣ����С C.��Ӧ������� D.��������ˮ
��T2��(T1>T2)ʱ����20L�����ܱ������г���2molNH3��2.75mo1O2��������ӦI.��Ӧ�����и����ʵ����ʵ�������ʱ��(t)�仯��ϵ��ͼ2��ʾ��T2��ʱ���÷�Ӧ��ƽ�ⳣ��K=_____��5minʱ���ı���ijһ������������ı����������Ϊ__________��