��Ŀ����
20��Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ����������о�����Ч���ƿ����еĵ������̼��������������ﺬ���Ե���Ϊ��Ҫ����1��������ȼ������ʱ������N2��O2�ķ�Ӧ��N2+O2�T2NO���ǵ�������β���к���NO��ԭ��֮һ����ѧ���������NH3�ڴ������½�NOx��ԭ��N2���ŷţ�
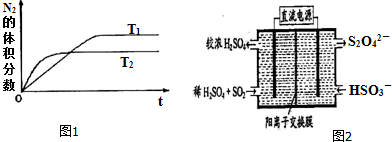
����T1��T2�¶��£�һ������NH3�����ֽⷴӦʱN2�����������ʱ��仯��ͼ1��ʾ������ͼ���жϷ�ӦN2��g��+3H2��g���T2NH3��g���ġ�H��0���������������
����T3�¶��£���2L�ܱ������г���10molN2��5mo1H2��50���ﵽƽ�⣬���NH3�����ʵ���Ϊ2mol����÷�Ӧ������v��N2��0.01mol•L-1•s-1�����¶��£�������ѹǿ�˷�Ӧ��ƽ�ⳣ�������䣨���������С���������䡱����ȷ������������ʼʱ�����������г���N2��H2��Ϊ10mol����ﵽƽ���H2��ת���ʽ����ͣ�������ߡ��������͡���
��2������ͼ2��ʾװ�ã��缫��Ϊ���Ե缫��������SO2���������ų�����Һ������NO2��
�������ĵ缫��ӦʽΪSO2+2H2O-2e-�TSO42-+4H+��
���ڼ��������£��������ų�����Һ����NO2��ʹ��ת��Ϊ�����壬ͬʱ��SO32-���ɣ��÷�Ӧ�����ӷ���ʽΪ4S2O42-+2NO2+8OH-�T8SO42-+N2+4H2O��
��3��һ�������¿��ü״���CO��Ӧ���ɴ�������CO��Ⱦ�������£���a mol•L-1�Ĵ�����b mol-L-1Ba��OH��2��Һ�������ϣ���ַ�Ӧ����Һ�д���2c��Ba2+��=c��CH3COO-������û����Һ�д���ĵ��볣��Ka=$\frac{2b}{a-2b}$��10-7L/mol���ú�a��b�Ĵ���ʽ��ʾ����
���� ��1���ٸ��ݡ��ȹ���ƽ��ֵ��ԭ���жϵ�T1��T2��С���ٸ���ƽ��ʱ��������������ж��¶ȶ�ƽ���Ӱ�죻
��v=$\frac{��c}{��t}$���پ�����֮�ȵ��ڻ�ѧ������֮�����㣻ƽ�ⳣ�������¶��йأ�����ʼʱ�����������г���N2��H2��Ϊ10mol���������ԭƽ��Ļ������ڳ�H25mol����Ȼƽ�������ƶ������ﵽƽ���H2��ת���ʽ����ͣ�
��2�������ݵ缫ԭ���ͷ�Ӧ�����е����ӱ仯д���缫��Ӧ��
�������ڼ��������£������ų�����ҺΪS2O42-�������������䷢����Ӧ4S2O42-+2NO2+8OH-�T8SO42-+N2+4H2O��
��3����Һ������������Ũ�ȼ���һ�룬�������ƽ�ⳣ����Ũ���أ���ϸ�����㣮
��� �⣺��1���ٸ���ͼ���жϣ�T2�����ȵ���ƽ�⣬��Ӧ���ʴ��¶Ƚϸߣ����¶����ߣ����������������С��˵�������¶�ƽ��������Ӧ�ƶ��������¶������ȷ�����У�������ӦΪ���ȷ�Ӧ����H��0��
�ʴ�Ϊ������
��v��NO��=$\frac{\frac{��n}{V}}{��t}$=$\frac{\frac{2mol}{2L}}{50s}$=0.02 mol•L-1•s-1��v��N2����v��NO��=1��2������v��N2��=0.01mol•L-1•s-1����Ϊƽ�ⳣ�������¶��йأ�����
������ѹǿ�˷�Ӧ��ƽ�ⳣ�������䣻����ʼʱ�����������г���N2��H2��Ϊ10mol���������ԭƽ��Ļ������ڳ�H25mol����Ȼƽ�������ƶ������ﵽƽ���H2��ת���ʽ����ͣ��ʴ�Ϊ��0.01mol•L-1•s-1�����䣻���ͣ�
��2��������ͼʾ��֪��������������Ϊ����������Զ����������ڵ���Ϊ��������������������ӦSO2-2e-+2H2O�TSO42-+4H+��
�ʴ�Ϊ��SO2+2H2O-2e-=SO32-+4H+��
�������ų�����ҺΪS2O42-�������������䷢����Ӧ��S2O42-����Ԫ����+3�ۣ���Ϊ���������Ԫ��Ϊ+4�ۣ�S2O42-�������ǻ�ԭ�������������еĵ�Ԫ�ػ��ϼ�Ϊ+4�۱�Ϊ����0�ۣ�������������ԭΪ������������ԭ���غ�͵�ʧ�����غ�ɵã����������ӷ���ʽΪ4S2O42-+2NO2+8OH-�T8SO42-+N2+4H2O���ʴ�Ϊ��4S2O42-+2NO2+8OH-�T8SO42-+N2+4H2O��
��3����Ӧƽ��ʱ��2c��Ba2+��=c��CH3COO-��=bmol/L���ݵ���غ㣬��Һ��c��H+��=c��OH-��=10-7mol/L����Һ�����ԣ��������ƽ�ⳣ�����ݵ��뷽��ʽд��K=$\frac{[CH{\;}_{3}COO{\;}^{-}]•[H{\;}^{+}]}{[CH{\;}_{3}COOH]}$=$\frac{b��10{\;}^{-7}}{\frac{a}{2}-b}$=$\frac{2b}{a-2b}$��10-7L/mol��
�ú�a��b�Ĵ���ʽ��ʾ�û����Һ�д���ĵ��볣��Ϊ$\frac{2b}{a-2b}$��10-7L/mol��
�ʴ�Ϊ��$\frac{2b}{a-2b}$��10-7L/mol��
���� ���⿼���Ȼ�ѧ����ʽ����ѧƽ�ⳣ������ѧƽ��Ӱ�����ط�����ԭ���ԭ���ķ���Ӧ�ã���Ҫ��������ʵ���ƽ�ⳣ���ļ��㣬��Ŀ�Ѷ��еȣ�

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�| A�� | �������ʯ��ʯ�ϣ�CaCO3+2H+�TCa2++H2O+CO2�� | |
| B�� | ����ϡ���ᷴӦ 2Fe+6H+=2Fe3++3H2�� | |
| C�� | ͭƬ������������Һ�У�Cu+2Ag+�T2Ag+Cu2+ | |
| D�� | ����ͭ������������Һ��Ӧ��Ba2++SO42-=BaSO4�� |
| A�� | 242.5kJ•mol-1 | B�� | 286.5kJ•mol-1 | C�� | 198.5kJ•mol-1 | D�� | 573kJ•mol-1 |

 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��