��Ŀ����
±����A��C2H5X����һ����ɫҺ�壬Ϊ̽��A�����ʣ��������ʵ�鷽����
����һ����A�м�����������Һ����������á�
����������A�мӹ���NaOHˮ��Һ����������ã���Һ��ֲ��ȡ��ˮ�㡱��Һ�������μ���������Һ��
����������A�м������NaOH�Ҵ���Һ�����ȣ���ַ�Ӧ��ȡ��Һ���������μ����Լ�B����������Һ���ð�ɫ������
����������Ϣ�ش����⡣
��1��C2H5X�е�X�� �� ��д��ѧʽ��
��2������һ�пɹ۲쵽�������� ��
��3��������Ϊ�������ﲻ������X-��ʵ��Ŀ�ģ������� ��
��4���������У��Լ�B�� ��д���������п��ܷ�����Ӧ�Ļ�ѧ����ʽ
����һ����A�м�����������Һ����������á�
����������A�мӹ���NaOHˮ��Һ����������ã���Һ��ֲ��ȡ��ˮ�㡱��Һ�������μ���������Һ��
����������A�м������NaOH�Ҵ���Һ�����ȣ���ַ�Ӧ��ȡ��Һ���������μ����Լ�B����������Һ���ð�ɫ������
����������Ϣ�ش����⡣
��1��C2H5X�е�X�� �� ��д��ѧʽ��
��2������һ�пɹ۲쵽�������� ��
��3��������Ϊ�������ﲻ������X-��ʵ��Ŀ�ģ������� ��
��4���������У��Լ�B�� ��д���������п��ܷ�����Ӧ�Ļ�ѧ����ʽ
(��14��)��1��Cl ��2�֣�
��2����Һ�ֲ㣨2�֣�
��3��������NaOH��Һ������������Һ��Ӧ���ɳ���(AgOH��Ag2O)���Ӷ�����X-�ļ��顣 ��2�֣�
��4��ϡ���ᣨ2�֣�CH3CH2Br+NaOH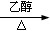 CH2=CH2��+NaBr+H2O ��2�֣�
CH2=CH2��+NaBr+H2O ��2�֣�
HNO3+NaOH=NaNO3+H2O ��2�֣� NaCl+AgNO3=AgCl��+NaNO3 ��2�֣�
��2����Һ�ֲ㣨2�֣�
��3��������NaOH��Һ������������Һ��Ӧ���ɳ���(AgOH��Ag2O)���Ӷ�����X-�ļ��顣 ��2�֣�
��4��ϡ���ᣨ2�֣�CH3CH2Br+NaOH
 CH2=CH2��+NaBr+H2O ��2�֣�
CH2=CH2��+NaBr+H2O ��2�֣�HNO3+NaOH=NaNO3+H2O ��2�֣� NaCl+AgNO3=AgCl��+NaNO3 ��2�֣�
�����������1�����ݼ�����������Һ���ð�ɫ������X��Cl��
��2������Ӧ�������۲쵽��Һ�ֲ������
��3��NaOH��Һ������������Һ��Ӧ���ɳ���(AgOH��Ag2O)���ʷ������ﲻ��ʵ��Ŀ�ġ�
��4��Ҫ����X���ӣ���Ҫ�к͵�����ļ�����Һ�����Լ�B��ϡ���ᡣ�����������ķ�ӦΪCH3CH2Br+NaOH
 CH2=CH2��+NaBr+H2O��HNO3+NaOH=NaNO3+H2O��NaCl+AgNO3=AgCl��+NaNO3��
CH2=CH2��+NaBr+H2O��HNO3+NaOH=NaNO3+H2O��NaCl+AgNO3=AgCl��+NaNO3���������������й�ʵ�鷽������ƺ����۵Ŀ��飬Ҫ��ѧ����Ϥ��ʵ������ݼ�ԭ�����ܹ�����ͬѧ�ǽ��з������⡢��������������

��ϰ��ϵ�д�
�����Ŀ

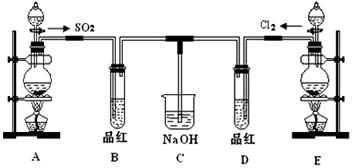
 MnCl2+Cl2��+2H2O���÷�Ӧ�еĻ�ԭ����____________���ѧʽ����
MnCl2+Cl2��+2H2O���÷�Ӧ�еĻ�ԭ����____________���ѧʽ����