��Ŀ����
ij��ѧʵ��С���ͬѧΪ��̽��SO2����ˮ��Ư���ԣ��������ʵ��װ�á�
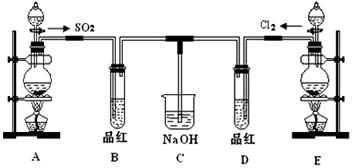
��1��Cװ�õ����ã�______________________________��
��2��ʵ������װ��E�Ʊ�Cl2����ѧ��Ӧ����ʽΪ��MnO2+4HCl��Ũ�� MnCl2+Cl2��+2H2O���÷�Ӧ�еĻ�ԭ����____________���ѧʽ����
MnCl2+Cl2��+2H2O���÷�Ӧ�еĻ�ԭ����____________���ѧʽ����
��3���ٷ�Ӧ��ʼһ��ʱ��۲쵽B��D�����Թ��е�Ʒ����Һ���ֵ������ǣ�
B_________________________�� D________________________��
��ֹͣͨ����,�ٸ�B��D�����Թֱܷ���ȣ������Թ��е�����ֱ�Ϊ
B_________________________�� D________________________��
��4����һ��ʵ��С���ͬѧ��ΪSO2����ˮ����Ư���ԣ�����Ϻ�Ư���Կ϶�����ǿ�����ǽ��Ƶõ�SO2��Cl2��1:1ͬʱͨ�뵽Ʒ����Һ�У��������Ʒ����Һδ��ɫ��������������������ԭ��_____________________________���û�ѧ����ʽ��ʾ����

��1��Cװ�õ����ã�______________________________��
��2��ʵ������װ��E�Ʊ�Cl2����ѧ��Ӧ����ʽΪ��MnO2+4HCl��Ũ��
 MnCl2+Cl2��+2H2O���÷�Ӧ�еĻ�ԭ����____________���ѧʽ����
MnCl2+Cl2��+2H2O���÷�Ӧ�еĻ�ԭ����____________���ѧʽ������3���ٷ�Ӧ��ʼһ��ʱ��۲쵽B��D�����Թ��е�Ʒ����Һ���ֵ������ǣ�
B_________________________�� D________________________��
��ֹͣͨ����,�ٸ�B��D�����Թֱܷ���ȣ������Թ��е�����ֱ�Ϊ
B_________________________�� D________________________��
��4����һ��ʵ��С���ͬѧ��ΪSO2����ˮ����Ư���ԣ�����Ϻ�Ư���Կ϶�����ǿ�����ǽ��Ƶõ�SO2��Cl2��1:1ͬʱͨ�뵽Ʒ����Һ�У��������Ʒ����Һδ��ɫ��������������������ԭ��_____________________________���û�ѧ����ʽ��ʾ����
��1��β������������SO2��Cl2��ֹ��Ⱦ������2�֣�
��2��HCl��2�֣�
��3����Ʒ����ɫ (2��)�� Ʒ����ɫ�� (2��)
��2��HCl��2�֣�
��3����Ʒ����ɫ (2��)�� Ʒ����ɫ�� (2��)
�����������1��Cװ�õ������dz�ȥB��ʣ���SO2��D��ʣ���Cl2����C��������β������������SO2��Cl2��ֹ��Ⱦ������
��2��MnO2+4HCl��Ũ��
 MnCl2+Cl2��+2H2O HCl��Ϊ��ԭ����ʧȥ���ӣ�����������Ӧ
MnCl2+Cl2��+2H2O HCl��Ϊ��ԭ����ʧȥ���ӣ�����������Ӧ��3��SO2�� Cl2����ʹƷ����ɫ �����Ⱥ�B����ɫ��Ʒ����Һ�ָֻ���ɫ��D��������������Ҫ����ΪSO2��ˮ�������ɵ��������Ư���Ծ��в��ȶ��ԣ����Ⱥ���Һ�ָֻ���ԭ������ɫ����D����ʵ�������ɵ�HClOʹƷ����Һ��ɫ�����Ⱥ���Һ����ɫ��
��4��Cl2��SO2��ˮ�������·������·�Ӧ��Cl2+SO2+2H2O

 2HCl+H2SO4 HCl��H2SO4����Ư���ԡ���2�֣�2����ˮ��Ư����
2HCl+H2SO4 HCl��H2SO4����Ư���ԡ���2�֣�2����ˮ��Ư����������SO2����ˮ��Ư��ԭ���Ǹ��л�ѧ��һ���ѵ㣬������ؿ���ѧ����ʵ��̽�������ͷ������������������ƽʱҪע��ѧ���۲죬�ද�ԡ�

��ϰ��ϵ�д�
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
�����Ŀ
 HCl��HClO��һ�����淴Ӧ�������ܽ��е��ķ�Ӧ���ҷ�Ӧ���ɵĴ�����(HClO)��һ�����Ա�̼�ỹҪ�����ᡣд������ĵ��뷽��ʽ��____________________��
HCl��HClO��һ�����淴Ӧ�������ܽ��е��ķ�Ӧ���ҷ�Ӧ���ɵĴ�����(HClO)��һ�����Ա�̼�ỹҪ�����ᡣд������ĵ��뷽��ʽ��____________________��