��Ŀ����
Ϊ��֤����ʵ�����Ʊ�Cl2�Ĺ����л���ˮ������HCl�ӷ���������ͬѧ�������ͼ��ʾ��ʵ��װ�ã���Ҫ��ش����⣺
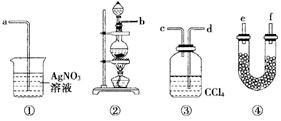
��1������ݼ�ͬѧ����ͼ��������Ӧ��װ�ã��ӿ�˳��b�� �� �� �� ��
��
��2��U�ι�����ʢ�Լ��Ļ�ѧʽΪ ��
��3��װ�â���CCl4�������� ��
��4����ͬѧ��Ϊ��ͬѧʵ����ȱ�ݣ�����֤������ͨ��AgNO3��Һ�е�����ֻ��һ�֣�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��AgNO3��Һ������ֻ��һ�֣���ͬѧ�����ij����װ��֮���ټ�װ�âݡ�����Ϊװ�â�Ӧ���� ֮�䣨��װ����ţ���ƿ�п��Է��� ��
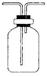
��5����ͬѧ������ͬѧ��Ƶ�װ�ú����������װ�ã�ֻ�轫ԭ���ձ��е�AgNO3��Һ����������Һ������Ϊ�ɽ���Һ���� ������۲쵽 ������֤����Cl2ʱ��HCl�ӷ�������

��1������ݼ�ͬѧ����ͼ��������Ӧ��װ�ã��ӿ�˳��b�� �� �� �� ��
��
��2��U�ι�����ʢ�Լ��Ļ�ѧʽΪ ��
��3��װ�â���CCl4�������� ��
��4����ͬѧ��Ϊ��ͬѧʵ����ȱ�ݣ�����֤������ͨ��AgNO3��Һ�е�����ֻ��һ�֣�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��AgNO3��Һ������ֻ��һ�֣���ͬѧ�����ij����װ��֮���ټ�װ�âݡ�����Ϊװ�â�Ӧ���� ֮�䣨��װ����ţ���ƿ�п��Է��� ��

��5����ͬѧ������ͬѧ��Ƶ�װ�ú����������װ�ã�ֻ�轫ԭ���ձ��е�AgNO3��Һ����������Һ������Ϊ�ɽ���Һ���� ������۲쵽 ������֤����Cl2ʱ��HCl�ӷ�������
��1��e f d c a����f e d c a��(2) ��ˮCuSO4 ��3������Cl2
��4���ۢ� ����KI��Һ ��5����ɫʯ����Һ ��ɫʯ����Һ��������ɫ
��4���ۢ� ����KI��Һ ��5����ɫʯ����Һ ��ɫʯ����Һ��������ɫ
�����������1������֤�Ʊ����������Ƿ���ˮ������Ȼ���ȥ�������ټ����Ƿ����Ȼ��⣬������Ӧ��װ�ýӿ�����˳��Ϊb��e��f��d��c��a��
��2����ˮ����ͭ��ˮ��Ӧ������ɫ��ˮ����ͭ���壬����������ˮ����U�ι�����ʢ�Լ��Ļ�ѧʽΪCuSO4��
��3���������������Ȼ�̼��Һ��������������������ֹ����HCl���顣
��4����װ����������HCl���壬Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�Ӧ�ų������ĸ��ţ��ڢ���������֮��Ҫ���������Ƿ���ȫ��ȥ��������������ǿ�����Ե����ʣ�����ʪ��ĵ���KI��ֽ���飬�粻��ɫ��˵���Ѿ���ȫ��ȥ��
��5��HCl�����ԣ�ʹ��ɫʯ����Һ��죬Cl2ʹ��ɫʯ����Һ�ȱ�����ɫ����ֻ��첻��ɫ��˵����Cl2ʱ��HCl�ӷ�������
�������������������Ʊ������ʵļ���Ϊ���壬����ѧ����ʵ�鷽��������ۣ���Ŀ�ۺ��Խ�ǿ�ϣ��Ѷ��еȡ�����ʱ�ؼ�����ʵ��ԭ����Ŀ�ļ���װ�õ����ã�����������ѧ���淶�Ͻ���ʵ����������ʹ���˼ά������

��ϰ��ϵ�д�
�����Ŀ
 2Cl2��2H2O����ʵ���ȵ�ѭ�����á�
2Cl2��2H2O����ʵ���ȵ�ѭ�����á�