��Ŀ����
10��ʵ���ҿ����ü״���������ͭ������ͭ���Ʊ���ȩ��ͼ�Ǽס�����λͬѧ��Ƶ�ʵ��װ�ã����ҵķ�Ӧװ�ú��ռ�װ����ͬ�������巢��װ�ò�ͬ��D����������ڷ�Ӧ����ʱʹ�ã��ֱ��������ʾ��
��ش�
��1��������װ�ý���ʵ�飬��B���з�����Ӧ�Ļ�ѧ����ʽΪ2CH3OH+O2$��_{��}^{ͭ}$2HCHO+2H2O��
��2��������װ�ý���ʵ�飬��B����Ӧװ������ͭ��B���з�����Ӧ�Ļ�ѧ����ʽΪCH3OH+CuO$\stackrel{��}{��}$HCHO+H2O��
��3��C�Թ���װ����Լ���ˮ�������ռ����������м�ȩ�ͼ״���
��4���Լס�����װ���е�A��B��C������Ҫ��ȡʲô��ʩ��ʵ�����ṩ��Ʒ��������ʹʵ��˳�����У�
��A��ˮԡ���ȣ�
��B�Ǿƾ��Ƽ��ȣ�
��C����ˮ��ȴ��
���� ��1����װ���в���ͨ���������״���������ͭ�Ĵ����������ɼ�ȩ��
��2����װ���в�ͨ������������Ҫ��������ͭ�����״���
��3����ȩ��״������ж����ʣ�Ӧ����β�����������߶�����ˮ��������ˮ���գ�
��4���״��ӷ�������ˮԡ���ȴٽ���ӷ����״�������Ӧ��Ҫ���ȣ��õ��ļ�ȩ�ӷ�������ˮ��ȴʹ��ת��ΪҺ�壮
��� �⣺��1���״������������ɼ�ȩ��ˮ����ӦΪ2CH3OH+O2$��_{��}^{ͭ}$2HCHO+2H2O��
�ʴ�Ϊ��2CH3OH+O2$��_{��}^{ͭ}$2HCHO+2H2O��
��2����װ������������ͭ�����״����ɼ�ȩ������ʽ��CH3OH+CuO$\stackrel{��}{��}$HCHO+H2O��
�ʴ�Ϊ������ͭ��CH3OH+CuO$\stackrel{��}{��}$HCHO+H2O��
��3����ȩ��״������ж����ʣ�Ӧ����β�����������߶�����ˮ��������ˮ���գ�
�ʴ�Ϊ��ˮ����ȩ�ͼ״���
��4���״��ӷ�������ˮԡ���ȴٽ���ӷ�������A����ˮԡ���ȣ�
�״�������Ӧ��Ҫ���ȣ�����B���þƾ��Ƽ��ȣ�
�õ��ļ�ȩ�ӷ�������C������ˮ��ȴʹ��ת��ΪҺ�壻
�ʴ�Ϊ��ˮԡ���ȣ��ƾ��Ƽ��ȣ���ˮ��ȴ��
���� ����Ϊʵ���⿼���˼�ȩ���Ʊ�����Ϥ���Ĵ�������Ӧ�ǽ���ؼ���ע�ⷴӦ��������������ʣ���Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 1 mol Al3+���еĺ��������Ϊ3��6.02��1023 | |
| B�� | ��58.5 g NaCl����1.00 Lˮ�У�����NaCl��Һ��Ũ��Ϊ1.00 mol•L-1 | |
| C�� | 2 mol SO2������������O2��һ�������·�Ӧ��ת�Ƶĵ�����Ϊ4��6.02��1023 | |
| D�� | �����£�100mL pH=1��������Һ�к��е�H+������Ϊ0.01��6.02��1023 |
| A�� | AgCl�ڱ���NaCl��Һ�е�KSP���ڴ�ˮ�е�KSPС | |
| B�� | KSP��AgCl����KSP��Ag2CrO4������˵��Ag2CrO4��AgCl������ˮ | |
| C�� | ��0.00lmol•L-1AgNO3��Һ����0.001 mol•L-1KC1��0.00lmol•L-1K2CrO4�����Һ�У��Ȳ���Ag2CrO4���� | |
| D�� | ͬAgCl������Һ�еμ�Ũ��ˮ������ܽ⣬����Ϊ�������������Ӷ�ʹAgCl���ܽ�ƽ�������ƶ� |
| A�� | 22.4LO2��һ������6.02��1023�������� | |
| B�� | ��8.0gNaOH����1Lˮ�У�������Һ��NaOH�����ʵ���Ũ��Ϊ0.2mol/L | |
| C�� | �����ӵ�������H2O�����ڱ�״���µ������22.4L | |
| D�� | �ڱ�״���£�20mLNH3��60mLO2�����ķ��Ӹ�����Ϊ1��3 |
| A�� | ��ȥ���е������壺����CCl4��ȡ���Һ | |
| B�� | ��ȥ�����е���ϩ��ͨ�����Ը��������Һ�� | |
| C�� | ��ȥCO2�е�����HCl���壺ͨ��ʢ�б���Na2CO3��Һ��ϴ��ƿ | |
| D�� | ��ȥFeCl2��Һ�е�����FeCl3����������Fe�ۺ���� |
| A�� | Ba2+��Na+��I-��ClO- | B�� | Mg2+��Cl-��CH3COO-��CO32- | ||
| C�� | K+��Cl-��Fe2+��NO3- | D�� | Ca2+��Cl-��Na+��Br- |
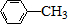
 A��B��C��D��E��Ϊ�л������A�ǻ�ѧʵ����������л����������ˮ����������ζ��B�IJ����ɺ���һ������ʯ�ͻ�����չ��ˮƽ���й����ʵ�ת����ϵ��ͼ����ʾ��
A��B��C��D��E��Ϊ�л������A�ǻ�ѧʵ����������л����������ˮ����������ζ��B�IJ����ɺ���һ������ʯ�ͻ�����չ��ˮƽ���й����ʵ�ת����ϵ��ͼ����ʾ�� CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O ��
��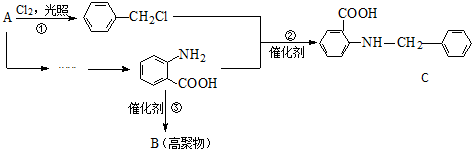
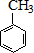 $��_{ˮԡ����}^{ŨH_{2}SO_{4}/ŨHNO_{3}}$
$��_{ˮԡ����}^{ŨH_{2}SO_{4}/ŨHNO_{3}}$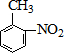
 $\stackrel{KMnO_{4}/H+}{��}$
$\stackrel{KMnO_{4}/H+}{��}$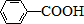
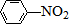 $\stackrel{Fe/HCl}{��}$
$\stackrel{Fe/HCl}{��}$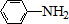 �������ԣ��ױ�������
�������ԣ��ױ������� ��
��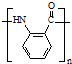 ��
��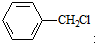 ˮ������ͬ���칹�壬�����Ȼ�����Һ������ɫ��Ӧ��X��3�ֽṹ��
ˮ������ͬ���칹�壬�����Ȼ�����Һ������ɫ��Ӧ��X��3�ֽṹ��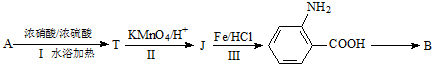
 ��
��