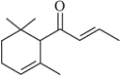
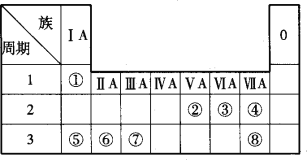
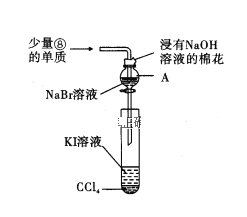
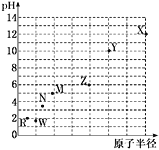
��Ŀ����
����Ŀ����֪���ȼ��a g��Ȳ����ʱ����1mol������̼�����Һ̬ˮ�����ų�����b kJ������Ȳȼ�յ��Ȼ�ѧ����ʽ��ȷ���ǣ���
A.2C2H2��g��+5O2��g��=4CO2��g��+2H2O��l������H=+2b kJ/mol
B.C2H2��g��+![]() O2��g��=2CO2��g��+H2O��l������H=-2b kJ/mol
O2��g��=2CO2��g��+H2O��l������H=-2b kJ/mol
C.2C2H2��g��+5O2��g��=4CO2��g��+2H2O��l������H=-2b kJ/mol
D.2C2H2��g��+5O2��g��=4CO2��g��+2H2O��l������H=-b kJ/mol
���𰸡�B
��������
��֪���ȼ��a g��Ȳ����ʱ����1mol������̼�����Һ̬ˮ��˵��a g��Ȳ�����ʵ���Ϊ0.5mol������ȫȼ�շų�����b kJ����1mol��Ȳ��ȫȼ�շų�����2b kJ������ȼ�յ��Ȼ�ѧ����ʽΪC2H2��g��+![]() O2��g��=2CO2��g��+H2O��l������H=-2b kJ/mol������ȷ����B��
O2��g��=2CO2��g��+H2O��l������H=-2b kJ/mol������ȷ����B��

����Ŀ��ij��ȤС���Է���м�Ƶ���������狀����������Ʊ���ˮ�ϲ�������(FeC2O4��2H2O)����һ���Ʊ��ߴ��Ȼ�ԭ���ۡ�
��֪��FeC2O4��2H2O������ˮ��150�濪ʼʧ�ᾧˮ��������H2C2O4Ϊ���壬������ˮ���ܽ�����¶����߶�����

��ش�
��1������ڣ�������Ӧ�����ӷ���ʽ____________________________���ò���H2C2O4�Թ�����Ҫ��Ϊ��_________________��
��2�����в�����������ȷ����_______________��
A������٣��ữ��Ҫ��Ϊ������Fe2+ˮ��
B������ۣ����������ˮϴ�ӿ���߳���Ч��
C������ۣ�����ڳ�ѹ�¿��ٸ���¶ȿ�ѡ���Ը���100��
��3����ȡһ������FeC2O4��2H2O�������������ܽ⣬����KMnO4�ζ����ⶨ�����������£�
n(Fe2��)/mol | n(C2O42��)��mol��1 | ������FeC2O4��2H2O���������� |
9.80��10��4 | 9.80��10��4 | 0.98 |
�ɱ��������Ʋ�����������Ҫ��������_________________��
��4��ʵ�ֲ���ܱ����õ�������������_________(��ѡ��������)
a��������b���ձ���c��������ƿ��d����ƿ��e��������f������¯
�ò���Ļ�ѧ����ʽ��______________________________________________��