��Ŀ����
����Ŀ����������(��Ҫ�ɷ�Ϊ�����Ͻ𣬺�����ͭ)Ϊԭ�ϣ�����NiO�IJ��ֹ����������£�
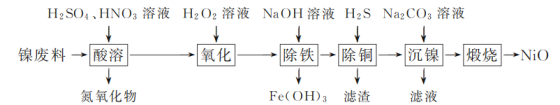
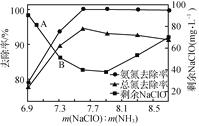
��֪���±��г��˼��ֽ������������������������pH(��ʼ������pH����������Ũ��Ϊ1.0 mol��L��1����)��
�������� | Fe(OH)3 | Fe(OH)2 | Ni(OH)2 |
��ʼ������pH | 1.5 | 6.5 | 7.7 |
������ȫ��pH | 3.3 | 9.9 | 9.2 |
(1) ��������ʱNiת��ΪNiSO4���ù������¶ȿ�����70��80 ���ԭ����________��
(2) ��������ʱ��Fe2���μӷ�Ӧ�����ӷ���ʽΪ________��
(3) ��������ʱ�������Һ��pH��ΧΪ________��
(4) ������������Ҫ�ɷ�Ϊ________(�ѧʽ)��
(5) ��������ʱ�õ���ʽ̼����[��xNiCO3��yNi(OH)2��ʾ]������
���ڸ������������£�����������ʽ̼�����õ�NiO���÷�Ӧ�Ļ�ѧ����ʽΪ________��
����������ʱ����ҺpH����ʽ̼������NiԪ�غ��������ӣ�ԭ����________��
���𰸡��¶ȵ���70 �棬��Ӧ���ʽ���,�¶ȸ���80 �棬HNO3�ֽ�(��ӷ�)�ӿ� 2Fe2����H2O2��2H��===2Fe3����2H2O 3.3��pH<7.7 CuS xNiCO3��yNi(OH)2![]() (x��y)NiO��xCO2����yH2O Ni(OH)2��Ni��������NiCO3��pHԽ��ʽ̼������Ni(OH)2��������
(x��y)NiO��xCO2����yH2O Ni(OH)2��Ni��������NiCO3��pHԽ��ʽ̼������Ni(OH)2��������
��������
������(��Ҫ�ɷ�Ϊ�����Ͻ𣬺�����ͭ)��ϡ���ᡢϡ��������������������������������ᣬͭҲ�����ᷴӦ�������������������ʱ����H2O2�ɽ�������������Һ�е�Fe2+����ΪFe3+�������������Ƶ�����ҺpH��ȥ�����ӣ����˵õ�����������������Һ������������Һ��ͨ��H2S���壬������ӦH2S+Cu2+=CuS��+2H+����ȥCu2+�������ͭ�����Һ�м���̼������Һ���������ӣ����ˡ�ϴ�ӡ�����õ��Ĺ��徭���յõ�NiO���ݴ˷�������
��1����������ʱ������������������Һ�������¶ȹ��ͣ����������л�ѧ��Ӧ���ʽϵͣ����¶ȹ��ߣ�����ֽ�ӿ죬�������ܽ�ͭ���ʴ�Ϊ�¶ȵ���70 �棬��Ӧ���ʽ���,�¶ȸ���80 �棬HNO3�ֽ�(��ӷ�)�ӿ죻
��2����������ʱ����H2O2�ɽ�������������Һ�е�Fe2+����ΪFe3+�����������ӷ���ʽΪ��2Fe2����H2O2��2H��===2Fe3����2H2O��
��3����������ʱҪ��������ȫ��������pH![]() 3.3�������Ӻ�ͭ����������Һ�У�pH<7.7�������������Һ��pH��ΧΪ3.3��pH<7.7��
3.3�������Ӻ�ͭ����������Һ�У�pH<7.7�������������Һ��pH��ΧΪ3.3��pH<7.7��
��4������������Һ��ͨ��H2S���壬������ӦH2S+Cu2+=CuS��+2H+����������ijɷ�ΪCuS��
��5�����ڸ������������£�����������ʽ̼�����õ�NiO��xNiCO3��yNi(OH)2�����·ֽ�����NiO��CO2��H2O���仯ѧ����ʽΪ��xNiCO3��yNi(OH)2![]() (x��y)NiO��xCO2����yH2O��
(x��y)NiO��xCO2����yH2O��
�ڼ�ʽ̼����[��xNiCO3��yNi(OH)2��ʾ]������Ni(OH)2��Ni��������NiCO3��pHԽ��ʽ̼������Ni(OH)2�����������Լ�ʽ̼������NiԪ�غ��������ӡ�

����Ŀ����H2O2��KI��ϴ�ྫ�����������������ʵ�飨��ʱ���ڲ���������ĭ����ijͬѧ�����������϶Ը�ʵ�����̽����
(1)����1��KI�ڸ÷�Ӧ�е����ã�H2O2 + I![]() = H2O + IO
= H2O + IO![]() ��H2O2 + IO
��H2O2 + IO![]() = H2O + O2��+ I
= H2O + O2��+ I![]() ���ܷ�Ӧ�Ļ�ѧ����ʽ��_______________________________________________��
���ܷ�Ӧ�Ļ�ѧ����ʽ��_______________________________________________��
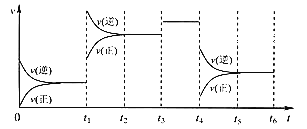
(2)����2��H2O2�ֽⷴӦ�����������仯��ͼ��ʾ�����Т���KI���룬����KI���롣�����ж���ȷ����______������ĸ����
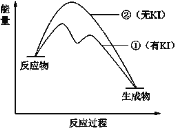
a������KI��ı��˷�Ӧ��·��
b������KI��ı����ܷ�Ӧ�������仯
c��H2O2 + I![]() = H2O + IO
= H2O + IO![]() �Ƿ��ȷ�Ӧ
�Ƿ��ȷ�Ӧ
(3)ʵ���з��֣�H2O2��KI��Һ��Ϻ����������ݣ���Һ��ɫ��ơ��ټ���CCl4�������ã��������Լ��١�
����3��I2Ҳ�ɴ�H2O2�ķֽⷴӦ��
�� ��CCl4�������úɹ۲쵽___________________________________��˵����I2���ɡ�
�� �������Լ��ٵ�ԭ������ǣ�
��. H2O2Ũ�Ƚ��ͣ�
��._________________________________________��
���¶���ʵ��˵����������Ҫԭ����H2O2��Һ�м���KI��Һ������Һ��ƺֳ����ȷ���A��B���Թ��С�A�Թܼ���CCl4��B�Թܲ���CCl4���ֱ������á��۲쵽��������________________________��
(4)����4��I![]() + I
+ I![]()
![]() I
I![]() K= 640��
K= 640��
Ϊ��̽����ϵ�к������Ĵ�����ʽ������ʵ�飺��20 mLһ��Ũ�ȵ�H2O2��Һ�м���10 mL 0.10 mol��L-1 KI��Һ����ƽ��������Ũ�����£�
�� | I | I | I |
Ũ��/ (mol��L-1) | 2.5��10-3 | a | 4.0��10-3 |
�� a =____________________��
�� ��ƽ����ϵ�г��˺���I![]() ��I
��I![]() ��I
��I![]() �⣬һ��������������������������_____________________��
�⣬һ��������������������������_____________________��