ΧβΡΩΡΎ»ί
Θ®10Ζ÷Θ§ΟΩΩ’2Ζ÷Θ©ΨωΕ®Έο÷ –‘÷ ΒΡ÷Ί“Σ“ρΥΊ «Έο÷ ΒΡΫαΙΙΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)“―÷ΣAΚΆBΈΣΒΎ»ΐ÷ήΤΎ‘ΣΥΊΘ§Τδ‘≠Ή”ΒΡΒΎ“Μ÷ΝΒΎΥΡΒγάκ»γœ¬±μΥυ ΨΘΚ
AΆ®≥Θœ‘____ΦέΘ§AΒΡΒγΗΚ–‘__ __BΒΡΒγΗΚ–‘(ΧνΓΑΘΨΓ±ΓΔΓΑΘΦΓ±ΜρΓΑΘΫΓ±)ΓΘ
(2)“―÷ΣΘΚ≤®≥ΛΈΣ300 nmΒΡΉœΆβΙβΒΡΙβΉ”ΥυΨΏ”–ΒΡΡήΝΩ‘ΦΈΣ399 kJΓΛmolΘ≠1ΓΘΗυΨίœ¬±μ”–ΙΊΒΑΑΉ÷ Ζ÷Ή”÷–÷Ί“ΣΜ·―ßΦϋΒΡ–≈œΔΘ§ΥΒΟς»ΥΧε≥Λ ±Φδ’’…δΉœΆβΙβΚσΤΛΖτ“Ή ή…ΥΚΠΒΡ‘≠“ρΘΚ
ΓΘ
(3)―–ΨΩΈο÷ ¥≈–‘±μΟςΘΚΫπ τ―τάκΉ”Κ§Έ¥≥…Ε‘ΒγΉ”‘ΫΕύΘ§‘ρ¥≈–‘‘Ϋ¥σΘ§¥≈Φ«¬Φ–‘Ρή‘ΫΚΟΓΘάκΉ”–Ά―θΜ·ΈοV2O5ΚΆCrO2÷–Θ§ ΚœΉς¬Φ“τ¥χ¥≈Ζέ‘≠ΝœΒΡ «________________ΓΘ
(4)Ρ≥≈δΚœΈοΒΡΖ÷Ή”ΫαΙΙ»γΆΦΥυ ΨΘ§ΤδΖ÷Ή”ΡΎ≤ΜΚ§”–__________(ΧνΉ÷ΡΗ)ΓΘ
AΘ°άκΉ”Φϋ BΘ°Ι≤ΦέΦϋ
CΘ°Ϋπ τΦϋ DΘ°≈δΈΜΦϋ EΘ°«βΦϋ
(1)“―÷ΣAΚΆBΈΣΒΎ»ΐ÷ήΤΎ‘ΣΥΊΘ§Τδ‘≠Ή”ΒΡΒΎ“Μ÷ΝΒΎΥΡΒγάκ»γœ¬±μΥυ ΨΘΚ
| ΒγάκΡή/kJΓΛmolΘ≠1 | I1 | I2 | I3 | I4 |
| A | 578 | 1 817 | 2 745 | 11 578 |
| B | 738 | 1 451 | 7 733 | 10 540 |
(2)“―÷ΣΘΚ≤®≥ΛΈΣ300 nmΒΡΉœΆβΙβΒΡΙβΉ”ΥυΨΏ”–ΒΡΡήΝΩ‘ΦΈΣ399 kJΓΛmolΘ≠1ΓΘΗυΨίœ¬±μ”–ΙΊΒΑΑΉ÷ Ζ÷Ή”÷–÷Ί“ΣΜ·―ßΦϋΒΡ–≈œΔΘ§ΥΒΟς»ΥΧε≥Λ ±Φδ’’…δΉœΆβΙβΚσΤΛΖτ“Ή ή…ΥΚΠΒΡ‘≠“ρΘΚ
ΓΘ
| Ι≤ΦέΦϋ | CΓΣC | CΓΣN | CΓΣS |
| ΦϋΡή/kJΓΛmolΘ≠1 | 347 | 305 | 259 |
(4)Ρ≥≈δΚœΈοΒΡΖ÷Ή”ΫαΙΙ»γΆΦΥυ ΨΘ§ΤδΖ÷Ή”ΡΎ≤ΜΚ§”–__________(ΧνΉ÷ΡΗ)ΓΘ

AΘ°άκΉ”Φϋ BΘ°Ι≤ΦέΦϋ
CΘ°Ϋπ τΦϋ DΘ°≈δΈΜΦϋ EΘ°«βΦϋ
.(1)+3
(2)ΉœΆβΙβΨΏ”–ΒΡΡήΝΩ±»ΒΑΑΉ÷ Ζ÷Ή”÷–÷Ί“ΣΒΡΜ·―ßΦϋCΓΣCΓΔCΓΣNΚΆCΓΣSΒΡΦϋΡήΕΦ¥σΘ§ΉœΆβΙβΒΡΡήΝΩΉψ“‘ Ι’β–©Μ·―ßΦϋΕœΝ―Θ§¥”ΕχΤΤΜΒΒΑΑΉ÷ Ζ÷Ή”
(3)CrO2 (4)AC
(2)ΉœΆβΙβΨΏ”–ΒΡΡήΝΩ±»ΒΑΑΉ÷ Ζ÷Ή”÷–÷Ί“ΣΒΡΜ·―ßΦϋCΓΣCΓΔCΓΣNΚΆCΓΣSΒΡΦϋΡήΕΦ¥σΘ§ΉœΆβΙβΒΡΡήΝΩΉψ“‘ Ι’β–©Μ·―ßΦϋΕœΝ―Θ§¥”ΕχΤΤΜΒΒΑΑΉ÷ Ζ÷Ή”
(3)CrO2 (4)AC
Θ®1Θ©AΒΡΒΎΥΡΒγάκΡή‘Ε¥σ”ΎΒΎ»ΐΒγάκΡήΘ§Υυ“‘A «ΒΎ»ΐ÷ήΤΎΒΡAlΘ§œ‘ΘΪ3ΦέΓΘB «ΟΨΘ§Ϋπ τ–‘‘Ϋ«ΩΘ§ΒγΗΚ–‘‘Ϋ–ΓΘ§Υυ“‘AΒΡΒγΗΚ–‘¥σ”ΎΟΨΒΡΓΘ
Θ®2Θ©ΗυΨίΦϋΡήΩ…÷ΣΘ§ΉœΆβΙβΨΏ”–ΒΡΡήΝΩ±»ΒΑΑΉ÷ Ζ÷Ή”÷–÷Ί“ΣΒΡΜ·―ßΦϋCΓΣCΓΔCΓΣNΚΆCΓΣSΒΡΦϋΡήΕΦ¥σΘ§ΉœΆβΙβΒΡΡήΝΩΉψ“‘ Ι’β–©Μ·―ßΦϋΕœΝ―Θ§¥”ΕχΤΤΜΒΒΑΑΉ÷ Ζ÷Ή”ΓΘ
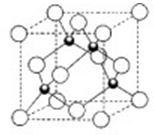
Θ®3Θ©άκΉ”–Ά―θΜ·ΈοV2O5ΚΆCrO2÷–Θ§Ϋπ τ―τάκΉ”Κ§Έ¥≥…Ε‘ΒγΉ”Ζ÷±π «0ΚΆ2Θ§Υυ“‘ ΚœΉς¬Φ“τ¥χ¥≈Ζέ‘≠ΝœΒΡ «CrO2 ΓΘ
Θ®4Θ©ΗυΨίΉι≥…‘ΣΥΊΩ…÷ΣΘ§»Ϊ≤ΩΕΦ «Ζ«Ϋπ τΘ§Υυ“‘ΟΜ”–άκΉ”ΦϋΚΆΫπ τΦϋΘ§¥πΑΗ―ΓACΓΘ
Θ®2Θ©ΗυΨίΦϋΡήΩ…÷ΣΘ§ΉœΆβΙβΨΏ”–ΒΡΡήΝΩ±»ΒΑΑΉ÷ Ζ÷Ή”÷–÷Ί“ΣΒΡΜ·―ßΦϋCΓΣCΓΔCΓΣNΚΆCΓΣSΒΡΦϋΡήΕΦ¥σΘ§ΉœΆβΙβΒΡΡήΝΩΉψ“‘ Ι’β–©Μ·―ßΦϋΕœΝ―Θ§¥”ΕχΤΤΜΒΒΑΑΉ÷ Ζ÷Ή”ΓΘ
Θ®3Θ©άκΉ”–Ά―θΜ·ΈοV2O5ΚΆCrO2÷–Θ§Ϋπ τ―τάκΉ”Κ§Έ¥≥…Ε‘ΒγΉ”Ζ÷±π «0ΚΆ2Θ§Υυ“‘ ΚœΉς¬Φ“τ¥χ¥≈Ζέ‘≠ΝœΒΡ «CrO2 ΓΘ
Θ®4Θ©ΗυΨίΉι≥…‘ΣΥΊΩ…÷ΣΘ§»Ϊ≤ΩΕΦ «Ζ«Ϋπ τΘ§Υυ“‘ΟΜ”–άκΉ”ΦϋΚΆΫπ τΦϋΘ§¥πΑΗ―ΓACΓΘ

ΝΖœΑ≤αœΒΝ–¥πΑΗ
 –Γ―ßΤΎΡ©≥ε¥Χ100Ζ÷œΒΝ–¥πΑΗ
–Γ―ßΤΎΡ©≥ε¥Χ100Ζ÷œΒΝ–¥πΑΗ ΤΎΡ©Η¥œΑΦλ≤βœΒΝ–¥πΑΗ
ΤΎΡ©Η¥œΑΦλ≤βœΒΝ–¥πΑΗ ≥§Ρή―ßΒδΒΞ‘ΣΤΎ÷–ΤΎΡ©Ή®Χβ≥ε¥Χ100Ζ÷œΒΝ–¥πΑΗ
≥§Ρή―ßΒδΒΞ‘ΣΤΎ÷–ΤΎΡ©Ή®Χβ≥ε¥Χ100Ζ÷œΒΝ–¥πΑΗ ΜΤΗ‘360Ε»Ε®÷ΤΟήΨμœΒΝ–¥πΑΗ
ΜΤΗ‘360Ε»Ε®÷ΤΟήΨμœΒΝ–¥πΑΗ
œύΙΊΧβΡΩ

 Θ§ΟΩΗωΨßΑϊ÷–Ζ÷Χ·2ΗωΦΊ‘≠Ή”
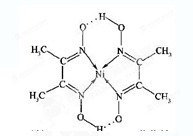
Θ§ΟΩΗωΨßΑϊ÷–Ζ÷Χ·2ΗωΦΊ‘≠Ή” Rx[CrCln(H2O)6Θ≠n]+xH+ΓΘΫΪΚ§0.0015 mol[CrCln(H2O)6Θ≠n]xΘΪΒΡ»ή“ΚΘ§”κR-HΆξ»ΪΫΜΜΜΚσΘ§÷–ΚΆ…ζ≥…ΒΡH+–η≈®Ε»ΈΣ0.1200 molΓΛL-1 NaOH»ή“Κ25.00 mLΘ§‘ρΗΟ≈δάκΉ”ΒΡΜ·―ß ΫΈΣ_______ΓΘ
Rx[CrCln(H2O)6Θ≠n]+xH+ΓΘΫΪΚ§0.0015 mol[CrCln(H2O)6Θ≠n]xΘΪΒΡ»ή“ΚΘ§”κR-HΆξ»ΪΫΜΜΜΚσΘ§÷–ΚΆ…ζ≥…ΒΡH+–η≈®Ε»ΈΣ0.1200 molΓΛL-1 NaOH»ή“Κ25.00 mLΘ§‘ρΗΟ≈δάκΉ”ΒΡΜ·―ß ΫΈΣ_______ΓΘ