��Ŀ����
����Ŀ�������£�ij�ݻ��̶����ܱ������ɿ��ƶ��Ļ�������A��B���ң���A���г���H2��O2�Ļ�����壬��B���г���3mol��������ʱ������λ����ͼ��ʾ��

��1��A�һ����������ʵ���Ϊ__________����״���µ����Ϊ_____________L ��
��2��ʵ����A�һ�����������Ϊ102g����û��������ܶ���ͬ��ͬѹ�����������ܶȵ� ______________����
��3������A����H2��O2�Ļ�������ȼ�������ָ�ԭ�¶Ⱥ����ջ���ͣ����λ����_______ �̶ȣ�����������ѹǿ�뷴Ӧǰ����ѹǿ֮��Ϊ _____________��
���𰸡� 6mol 134.4 8.5 2 1��2
����������1��ͬ��ͬѹ�£���������ʵ���֮�ȵ������֮�ȣ���A�����������ʵ���Ϊ3mol��![]() =6mol����״���µ����Ϊ6mol��22.4 Lmol-1=134.4L��
=6mol����״���µ����Ϊ6mol��22.4 Lmol-1=134.4L��
��2��A���л�������ƽ��Ħ������Ϊ��102g��6mol=17g��mol-1������Ħ��������2g��mol-1����Ϊͬ��ͬѹ��������ܶ�֮�ȵ���Ħ������֮�ȣ��ʸû��������ܶ���ͬ��ͬѹ�����������ܶȵ�8.5����
��3�����������ʵ���Ϊxmol���������ʵ���Ϊymol����x+y=6��2x+32y=102�����x=3��y=3�����Խ�A����H2��O2�Ļ�������ȼ�������ָ������º�ʣ������1.5mol������ΪB����3mol�������������ջ���ͣ����λ����2�̶���ԭ�ȹ���9mol���壬���ڹ���4.5mol���壬����PV=nRT��ͬ��ͬ�������ѹǿ֮�ȵ������ʵ���֮�ȣ�������������ѹǿ�뷴Ӧǰ����ѹǿ֮��Ϊ1:2��

����Ŀ����Ҫ��ش����⣺
��1��KAl(SO4)2�ĵ��뷽��ʽ_________��
��2����ʯ����Һ��ͨ����������ʼʱ��Һ��죬һ��ʱ�����Һ��ɫ����ʹ��Һ������ɫ�����ֱ���_________��_________ (��������)��
��3��ʵ����������0.5mol/L��������Һ480mL������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����___������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ���������________��
��4����ѧ����ʽH2S+H2SO4(Ũ)��SO2��+S��+2H2O��������4.48L(���)SO2ʱ��ת�Ƶĵ�����__________mol��
��5�������ĸ�ͼ���У��������ʾ�������ʵ����ʵ������������ʾ���ɳ�����������������������A��D��ѡ����ϸ���Ҫ���������˱��С�
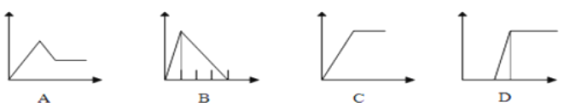
��Һ | ��������� | ��� |
��AlCl3��Һ | ͨ�������NH3 | ___ |
��Na2CO3��NaOH����Һ | ������������� | ___ |
��NaAlO2��Һ | �μ�ϡ���������� | __ |
����Ŀ�����仯������������;��������ʵ������������±���ʾ��
H2S | S8 | FeS2 | SO2 | SO3 | H2SO4 | |
�۵�/�� | 85.5 | 115.2 | >600���ֽ⣩ | 75.5 | 16.8 | 10.3 |
�е�/�� | 60.3 | 444.6 | 10.0 | 45.0 | 337.0 |
�ش��������⣺
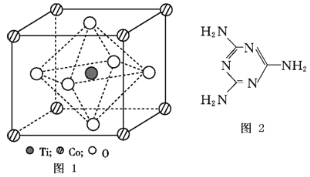
��1����̬Feԭ�Ӽ۲���ӵĵ����Ų�ͼ���������ʽ��Ϊ__________����̬Sԭ�ӵ���ռ������ܼ��ĵ���������ͼΪ_________�Ρ�
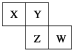
��2�����ݼ۲���ӶԻ������ۣ�H2S��SO2��SO3����̬�����У�����ԭ�Ӽ۲���Ӷ�����ͬ���������ӵ���_________��
��3��ͼ��a��ΪS8�Ľṹ�����۵�ͷе�Ҫ�ȶ���������۵�ͷе�ߺܶ࣬��Ҫԭ��Ϊ__________��

��4����̬���������Ե�������ʽ���ڣ�����ӵ����幹��Ϊ_____�Σ����й��ۼ���������______�֣��������������д�����ͼ��b����ʾ�����۷��ӣ��÷�����Sԭ�ӵ��ӻ��������Ϊ________��
��5��FeS2����ľ�����ͼ��c����ʾ�������߳�Ϊa nm��FeS2���ʽ��ΪM�������ӵ�������ֵΪNA���侧���ܶȵļ������ʽΪ___________g��cm3��������Fe2+λ��![]() ���γɵ�������������ģ�����������ı߳�Ϊ______nm��
���γɵ�������������ģ�����������ı߳�Ϊ______nm��