��Ŀ����
����Ŀ����Ҫ��ش����⣺
��1��KAl(SO4)2�ĵ��뷽��ʽ_________��
��2����ʯ����Һ��ͨ����������ʼʱ��Һ��죬һ��ʱ�����Һ��ɫ����ʹ��Һ������ɫ�����ֱ���_________��_________ (��������)��
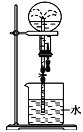
��3��ʵ����������0.5mol/L��������Һ480mL������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����___������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ���������________��

��4����ѧ����ʽH2S+H2SO4(Ũ)��SO2��+S��+2H2O��������4.48L(���)SO2ʱ��ת�Ƶĵ�����__________mol��
��5�������ĸ�ͼ���У��������ʾ�������ʵ����ʵ������������ʾ���ɳ�����������������������A��D��ѡ����ϸ���Ҫ���������˱��С�
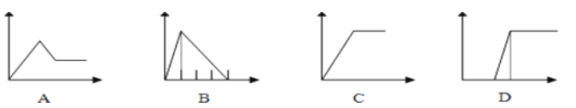
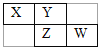
��Һ | ��������� | ��� |
��AlCl3��Һ | ͨ�������NH3 | ___ |
��Na2CO3��NaOH����Һ | ������������� | ___ |
��NaAlO2��Һ | �μ�ϡ���������� | __ |
���𰸡�KAl(SO4)2��K++Al3++2SO42- H+ HClO A��C �ձ�����������500mL����ƿ 0.4 C D B
��������
��1��KAl(SO4)2��ˮ��Һ�е���������ӡ������ӡ���������ӣ����뷽��ʽ��KAl(SO4)2��K++Al3++2SO42-��
��2����ʯ����Һ��ͨ����������ʼʱ��Һ��죬һ��ʱ�����Һ��ɫ��ʹʯ����Һ������ɫ�����ֱ���H+��HClO��
��3�����Ʋ�����Ҫ�м��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����������Ͳȷ��ȡŨ���ᵹ��ʢ��ˮ���ձ��У�ʹ�����ܽ⣬����ȴ�����£���ϡ�����ز�����ע��500mL������ƿ�У�������ˮϴ���ձ���������2��3�Σ�ϴ��Һ��ע������ƿ������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm�������ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶����У�������ƿ�ǽ�����ҡ�ȼ��ɣ����Բ���Ҫ����������ƿ����Һ©��������Ҫ���������ձ�����������500mL����ƿ��
��4��H2S+H2SO4(Ũ)��SO2��+S��+2H2O��H2SO4�Ļ�ԭ������SO2��ÿ����1mol SO2ת��2mol���ӣ�������4.48L(���)SO2ʱ��ת�Ƶĵ�����![]() 0.4mol��
0.4mol��
��5����AlCl3��Һ�е��������ˮ�������������������Ȼ�泥���Ӧͼ����C����Na2CO3��NaOH����Һ����������ᣬ���η�����ӦOH-+H+��H2O��CO32-+2H+��HCO3-��HCO3-+H+��H2O+CO2������Ӧͼ����D����NaAlO2��Һ�μ�ϡ���������������η�����ӦAlO2-+H++H2O��Al(OH)3����Al(OH)3+3H+��Al3++3H2O����Ӧͼ����B��

 �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�