ÌâÄżÄÚÈĘ
ĄŸÌâÄżĄżŁš1Ł©Äł»ìșÏÈÜÒșÖĐÖ»șŹÓĐÏÂÁĐŒžÖÖÀëŚÓŁšČ»żŒÂÇËź”Ä”çÀ룩ŁșNaŁ«ĄąMg2Ł«ĄąAl3Ł«ĄąClŁĄąSO42ŁŁŹÈôNaŁ«ĄąMg2Ł«ĄąClŁĄąSO42Ł”ÄÎïÖÊ”ÄÁżĆš¶ÈÒÀŽÎÎȘ 0.2 mol/LĄą0.25 mol/LĄą0.5 mol/LĄą0.25mol/LŁŹÔò cŁšAl3Ł«Ł©=________ ĄŁ
Łš2Ł© ÄłÎïÖÊ A ŒÓÈÈʱ°Ž»ŻŃ§·œłÌÊœ 2AšT2B+C+3D ·ÖœâŁŹČúÎïŸùÎȘÆűÌ棏Čâ”ĂÓÉÉúłÉÎïŚéłÉ”Ä»ì șÏÎïÆűÌć¶Ô H2 ”ÄÏà¶ÔĂܶÈÎȘ 20ŁŹÔò·ŽÓŠÎï A ”ÄÄŠ¶ûÖÊÁżÎȘ_____ ĄŁ
Łš3Ł©ÔÚ±êŚŒŚŽżöÏÂŁŹœ« VL A ÆűÌ棚Ċ¶ûÖÊÁżÎȘ Mg/molŁ©ÈÜÓÚ 0.1L ËźÖĐŁŹËù”ĂÈÜÒș”ÄĂܶÈÎȘŠŃg/cm3ŁŹÔòŽËÈÜÒș”ÄÎïÖÊ”ÄÁżĆš¶ÈÎȘ_________molĄ€LŁ1
AŁź1000VŠŃ/(MVŁ«22400)molĄ€LŁ1 BŁźVŠŃ/(MVŁ«22400)molĄ€LŁ1
C.100VŠŃM/(MVŁ«22400)molĄ€LŁ1 D.MV/22.4(VŁ«0.1) ŠŃmolĄ€LŁ1
Łš4Ł©ÉúÌŹĆ©Ò”ÉæŒ°Ć©ŒÒ·ÊÁÏ”ÄŚÛșÏÀûÓĂ.ÄłÖÖ·ÊÁÏŸ·ąœÍ”Ă”œÒ»ÖÖșŹÓĐŒŚÍ饹¶țŃő»ŻÌŒĄą”ȘÆű ”Ä»ìșÏÆűÌć 8.96L(±êŚŒŚŽżö).žĂÆűÌćÍščęÊąÓĐșìÉ« CuO ·ÛÄ©”ÄÓČÖÊČŁÁ§čÜŁŹ·ąÉú”Ä·ŽÓŠÎȘŁșCH4Ł«4CuO![]() CO2ĄüŁ«2H2OĄüŁ«4CuŁŹ.”±ŒŚÍéÍêÈ«·ŽÓŠșóŁŹÓČÖÊČŁÁ§čÜ”ÄÖÊÁżŒőÇáÁË 4.8gĄŁœ«·ŽÓŠșóÆűÌćÍšÈë 2L 0.1mol/L ”ÄłÎÇć Ca(OH)2 ÈÜÒșŁŹłä·ÖÎüÊŐŁŹÉúłÉłÁ”í 10g.ÇóŁș
CO2ĄüŁ«2H2OĄüŁ«4CuŁŹ.”±ŒŚÍéÍêÈ«·ŽÓŠșóŁŹÓČÖÊČŁÁ§čÜ”ÄÖÊÁżŒőÇáÁË 4.8gĄŁœ«·ŽÓŠșóÆűÌćÍšÈë 2L 0.1mol/L ”ÄłÎÇć Ca(OH)2 ÈÜÒșŁŹłä·ÖÎüÊŐŁŹÉúłÉłÁ”í 10g.ÇóŁș
ąÙÔ»ìșÏÆűÌćÖĐŒŚÍé”ÄÌć»ę(±êŚŒŚŽżö)___________________________________________________
ąÚ·ŽÓŠșó”ÄÆűÌćÖĐ CO2 ”ÄÎïÖÊ”ÄÁżÎȘ_______________________
Łš5Ł©ÎȘÈ·¶šžőŒŰ·Ż xK2SO4Ą€yCr2(SO4)3Ą€zH2O”Ä·ÖŚÓÊœŁŹĆäłÉșŹŽËÎïÖÊ 31.28g ”ÄÈÜ Òș 400mLŁŹÈĄ 200mL ”ÄÈÜÒșŒÓÈë 1mol/L ”ÄBa(NO3)2ÈÜÒș 100mLŁŹÉúłÉłÁ”íŁŹčęÂËșóÂËÒșÖĐ ŒÓÈë 0.1mol/L H2SO4 ÈÜÒșŁŹÏûșÄ 200mL ÁòËáʱłÁ”íÇĄșĂÍêÈ«ŁŹÓĂčęÁż°±ËźŽŠÀíÊŁÓà”Ä 200mL ÈÜÒșŁŹÉúłÉ Cr(OH)3 łÁ”í 4.12gŁŹÊÔÈ·¶š xĄąyĄąz ”ÄÖ”.____________________________________________
ĄŸŽđ°žĄż1mol/L120g/molA1.68L0.1mol »ò 0.3molx=1ŁŹy=1ŁŹz=12
ĄŸœâÎöĄż
Łš1Ł©NaŁ«ĄąMg2Ł«ĄąClŁĄąSO42Ł”ÄÎïÖÊ”ÄÁżĆš¶ÈÒÀŽÎÎȘ 0.2 mol/LĄą0.25 mol/LĄą0.5 mol/LĄą0.25mol/LŁŹžùŸĘÈÜÒș”çșÉÊŰșăÓĐ3cŁšAl3Ł«Ł©+cŁšNa+Ł©+2cŁšMg2+Ł©=cŁšCl-Ł©+2cŁšSO42-Ł©ŁŹčÊŁș3cŁšAl3Ł«Ł©+ 0.2 mol/L +2ĄÁ0.25 mol/L =0.5 mol/L +2ĄÁ0.25mol/LŁŹœâ”ĂŁșcŁšAl3Ł«Ł©=1mol/LŁ»
Łš2Ł©ÁîA”ÄÎïÖÊ”ÄÁżÎȘ2molŁŹÓÉ·œłÌÊœ2A=2B+C+3DŁŹżÉÖȘ»ìșÏÆűÌćŚÜÎïÖÊ”ÄÁżÎȘ2mol+1mol+3mol=6molŁŹ»ìșÏÆűÌć¶ÔÇâÆű”ÄÏà¶ÔĂܶÈÎȘ20ŁŹÔò»ìșÏÆűÌćÆœŸùÄŠ¶ûÖÊÁż=2g/molĄÁ20=40g/molŁŹ»ìșÏÆűÌćŚÜÖÊÁż=6molĄÁ40g/molŁŹÓÉÖÊÁżÊŰș㶚ÂÉżÉÖȘA”ÄÖÊÁż”ÈÓÚ»ìșÏÆűÌćŚÜÖÊÁżŁŹčÊA”ÄÄŠ¶ûÖÊÁż=6molĄÁ40g/mol2mol=120g/molŁ»
Łš3Ł©VLÆűÌć”ÄÎïÖÊ”ÄÁż=![]() =
=![]() molŁŹÆűÌć”ÄÖÊÁż=
molŁŹÆűÌć”ÄÖÊÁż=![]() molĄÁMg/mol=
molĄÁMg/mol=![]() gŁŹ0.1LËź”ÄÖÊÁż=100mLĄÁ1g/mL=100gŁŹčÊVL AÆűÌćÈÜÓÚ0.1LËźÖĐŁŹËù”ĂÈÜÒșÖĐÈÜÖÊ”ÄÖÊÁż·ÖÊę=
gŁŹ0.1LËź”ÄÖÊÁż=100mLĄÁ1g/mL=100gŁŹčÊVL AÆűÌćÈÜÓÚ0.1LËźÖĐŁŹËù”ĂÈÜÒșÖĐÈÜÖÊ”ÄÖÊÁż·ÖÊę=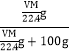 ĄÁ100%=
ĄÁ100%=![]() %ŁŹËù”ĂÈÜÒșĂܶÈÎȘŠŃg/cm3ŁŹčÊÈÜÒșÎïÖÊ”ÄÁżĆš¶È=
%ŁŹËù”ĂÈÜÒșĂܶÈÎȘŠŃg/cm3ŁŹčÊÈÜÒșÎïÖÊ”ÄÁżĆš¶È=![]() mol/L=
mol/L=![]() mol/LĄŁŽđ°žŃĄAŁ»
mol/LĄŁŽđ°žŃĄAŁ»
Łš4Ł©ąÙ±êżöÏÂŁŹ»ìșÏÆűÌć”ÄŚÜÎïÖÊ”ÄÁżÎȘ![]() =0.4molŁŹÉèŒŚÍéÖÊÁżÎȘmŁŹÉúłÉ¶țŃő»ŻÌŒÎȘnŁŹÔòŁș
=0.4molŁŹÉèŒŚÍéÖÊÁżÎȘmŁŹÉúłÉ¶țŃő»ŻÌŒÎȘnŁŹÔòŁș
CH4+4CuO![]() CO2Ąü+2H2OĄü+4Cu ÖÊÁżŒőÉÙ
CO2Ąü+2H2OĄü+4Cu ÖÊÁżŒőÉÙ
16g 44g 64g
m n 4.8g
m=![]() =1.2gŁŹn=
=1.2gŁŹn=![]() =3.3gŁŹ
=3.3gŁŹ
ÔònŁšCH4Ł©=![]() =0.075molŁŹVŁšCH4Ł©=0.075molĄÁ22.4L/mol=1.68LŁŹ
=0.075molŁŹVŁšCH4Ł©=0.075molĄÁ22.4L/mol=1.68LŁŹ
ąÚÉúłÉłÁ”í10gÎȘÌŒËážÆŁŹÌŒËážÆÎïÖÊ”ÄÁżÎȘ![]() =0.1molŁŹžùŸĘ·ŽÓŠCO2+CaCO3+H2O=Ca(HCO3)2ŁŹÈôčęÁż”ĶțŃő»ŻÌŒœ«Čż·ÖÌŒËážÆÈÜœâŁŹÔòÍšÈë”ĶțŃő»ŻÌŒ”ÄÎïÖÊ”ÄÁżÎȘ0.3molĄŁčÊ·ŽÓŠșó”ÄÆűÌćÖĐ CO2 ”ÄÎïÖÊ”ÄÁżÎȘ0.1mol »ò 0.3molŁ»
=0.1molŁŹžùŸĘ·ŽÓŠCO2+CaCO3+H2O=Ca(HCO3)2ŁŹÈôčęÁż”ĶțŃő»ŻÌŒœ«Čż·ÖÌŒËážÆÈÜœâŁŹÔòÍšÈë”ĶțŃő»ŻÌŒ”ÄÎïÖÊ”ÄÁżÎȘ0.3molĄŁčÊ·ŽÓŠșó”ÄÆűÌćÖĐ CO2 ”ÄÎïÖÊ”ÄÁżÎȘ0.1mol »ò 0.3molŁ»
Łš5Ł©ÓÉÓÚÉúČú”ÄCr(OH)3łÁ”í”ÄÖÊÁżÎȘ4.12gŁŹčÊÆäÎïÖÊ”ÄÁżn=![]() =0.04molŁŹžùŸĘžőÀëŚÓ”ÄÊŰșăżÉÖȘžőŒŰ·°ÖĐșŹÓĐ”ÄCr2(SO4)3”ÄÎïÖÊ”ÄÁżn=0.02molŁź0.02mol Cr2(SO4)3”ÄÖĐșŹÓĐÁòËážùÎȘ0.06molŁŹÓÉÓÚžőŒŰ·°șÍÁòËáčČÏûșÄ”ÄÏőËá±””ÄÎïÖÊ”ÄÁżÎȘ0.1molŁŹžùŸĘÁòËážù”ÄÊŰșăżÉÖȘŁŹÁòËáŒŰ”ÄÎïÖÊ”ÄÁżn=1mol/LĄÁ0.1L-0.1mol/LĄÁ0.2L-0.06mol=0.02molĄŁčÊžőŒŰ·°ÖĐșŹÓĐ”ÄËź”ÄÖÊÁżm=
=0.04molŁŹžùŸĘžőÀëŚÓ”ÄÊŰșăżÉÖȘžőŒŰ·°ÖĐșŹÓĐ”ÄCr2(SO4)3”ÄÎïÖÊ”ÄÁżn=0.02molŁź0.02mol Cr2(SO4)3”ÄÖĐșŹÓĐÁòËážùÎȘ0.06molŁŹÓÉÓÚžőŒŰ·°șÍÁòËáčČÏûșÄ”ÄÏőËá±””ÄÎïÖÊ”ÄÁżÎȘ0.1molŁŹžùŸĘÁòËážù”ÄÊŰșăżÉÖȘŁŹÁòËáŒŰ”ÄÎïÖÊ”ÄÁżn=1mol/LĄÁ0.1L-0.1mol/LĄÁ0.2L-0.06mol=0.02molĄŁčÊžőŒŰ·°ÖĐșŹÓĐ”ÄËź”ÄÖÊÁżm=![]() -mŁšK2SO4Ł©-m[Cr2ŁšSO4Ł©3Ł©]=15.64g-0.02molĄÁ174g/mol-0.02molĄÁ392g/mol=4.32gŁŹÔòËź”ÄÎïÖÊ”ÄÁżn=
-mŁšK2SO4Ł©-m[Cr2ŁšSO4Ł©3Ł©]=15.64g-0.02molĄÁ174g/mol-0.02molĄÁ392g/mol=4.32gŁŹÔòËź”ÄÎïÖÊ”ÄÁżn=![]() =0.24molŁŹčÊxŁșyŁșz=0.02molŁș0.02molŁș0.24mol=1Łș1Łș12ŁŹčÊx=1ŁŹy=1ŁŹz=12Łź
=0.24molŁŹčÊxŁșyŁșz=0.02molŁș0.02molŁș0.24mol=1Łș1Łș12ŁŹčÊx=1ŁŹy=1ŁŹz=12Łź

 ÌìÌìÏòÉÏÒ»±ŸșĂŸíÏ”ÁĐŽđ°ž
ÌìÌìÏòÉÏÒ»±ŸșĂŸíÏ”ÁĐŽđ°ž ХѧÉú10·ÖÖÓÓŠÓĂÌâÏ”ÁĐŽđ°ž
ХѧÉú10·ÖÖÓÓŠÓĂÌâÏ”ÁЎ𰞥ŸÌâÄżĄżÔÚ2 LĂܱŐÈĘÆśÖĐŁŹŒÓÈë”ÈÎïÖÊ”ÄÁż”ÄNOșÍO2ŁŹ800Ąæʱ·ąÉú·ŽÓŠŁș2NO(g)Ł«O2(g)Ąú2NO2(g)ŁŹÆäÖĐn(NO)ËæʱŒä”ı仯Èç±íŁș
ʱŒä/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)/mol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
Łš1Ł©ÉÏÊö·ŽÓŠ________ŁšÌÊÇĄ±»òĄ°Č»ÊÇĄ±Ł©żÉÄæ·ŽÓŠĄŁ
Łš2Ł©ÈçÍŒËùÊŸŁŹ±íÊŸNO2±ä»ŻÇúÏß”ÄÊÇ______ĄŁ
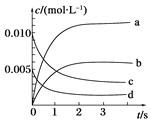
Łš3Ł©ÓĂO2±íÊŸŽÓ0Ą«2sÄڞ÷ŽÓŠ”ÄÆœŸùËÙÂÊvŁœ________ŁŹ5sʱO2”ÄŚȘ»ŻÂÊÎȘ_____________ĄŁ
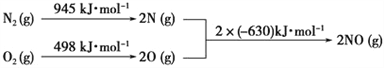
Łš4Ł©NOxÊÇÆûł”ÎČÆűÖĐ”ÄÖśÒȘÎÛÈŸÎïÖźÒ»ĄŁÆûł”·ą¶Ż»ú而śÊ±»áÒę·ąN2șÍO2·ŽÓŠŁŹÆäÄÜÁż±ä»ŻÈçÍŒËùÊŸĄŁĐŽłöžĂ·ŽÓŠ”ÄÈÈ»ŻŃ§·œłÌÊœŁș_______________________ĄŁ
Łš5Ł©ÒÔN2șÍH2ÎȘ·ŽÓŠÎïŁŹŃÎËáËữ”ÄNH4ClÈÜÒșÎȘ”çœâÖÊ”ÄÔ”çłŰŁŹč€ŚśÔÀíÈçÏÂÍŒËùÊŸŁŹÏÂÁĐË”·šČ»ŐęÈ·”ÄÊÇ___________ĄŁ
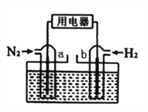
AŁźb”猫ÎȘžșŒ«
BŁź·ŽÓŠčęłÌÖĐŁŹÈÜÒșÖĐ”ÄCl-Ïòa”猫ÒƶŻ
CŁźa”猫”Ĕ猫·ŽÓŠÊœÎȘŁșN2+6e-+8H+=2NH4+
DŁź”çłŰ·ŽÓŠÎȘN2+3H2+2HCl=2NH4Cl
ĄŸÌâÄżĄżĐËÈ€ĐĄŚéÔÚÊ”ŃéÊÒÓĂÖƱž”ÄÂÈÆűÓëÒ»Ńő»Ż”ȘÔÚłŁÎÂłŁŃčÏÂșÏłÉŃÇÏőőŁÂÈĄŁ
ĄŸČéÔÄŚÊÁÏĄż ŃÇÏőőŁÂÈ(NOCl,ÈÛ”ăŁș-64.5 Ąæ,·Đ”ăŁș-5.5 Ąæ) ÊÇÒ»ÖÖ»ÆÉ«ÆűÌ棏ÒșÌćŚŽÌŹłÊșìșÖÉ«ŁŹÓöËźÒŚËźœâĄŁżÉÓĂÓÚșÏłÉÇćœàŒÁĄąŽ„ĂœŒÁŒ°ÖĐŒäÌć”ÈĄŁ¶ÔŃÛŸŠĄąÆ€·ôșÍճĀÓĐÇżÁÒŽÌŒ€ĐÔŁŹŸßÓĐÀàËÆÂÈÆűșÍ”ȘŃő»ŻÎï”ĶŸŚśÓĂĄŁ±ùËźÖĐŒÓÈëNaClżÉœ””ÍÎÂ¶ÈĄŁ
ĄŸÔÁÏÖƱžĄż ÔÚÊ”ŃéÊÒ·Ö±đÖƱžÔÁÏÆűNOșÍCl2ĄŁ
Łš1Ł©ÓĂÈçÏÂŚ°ÖĂÖƱžŽżŸ»žÉÔï”ÄÆűÌ棏ÇëČčłäϱíÖĐžśÒÇÆśÖĐ”ÄÊÔŒÁĄŁ
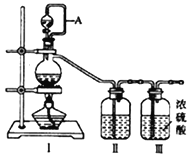
ÔÁÏÖƱž | Ś°ÖĂąń | Ś°ÖĂąń | Ś°ÖĂąò |
ÉŐÆżÖĐ | ·ÖÒș©¶·ÖĐ | ||
ÖƱžŽżŸ»Cl2 | MnO2 | ąÙ__________ | ąÚ______ |
ÖƱžŽżŸ»NO | Cu | ąÛ_______ | ąÜ_________ |
ĄŸșÏłÉŃÇÏőőŁÂÈĄż ÀûÓĂÖÆ”Ă”ÄNOșÍCl2ÖƱžNOCl,Ś°ÖĂÈçÍŒËùÊŸŁș
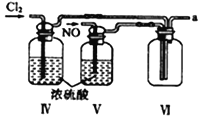
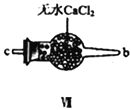
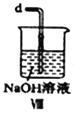
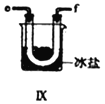
Łš2Ł©Ś°ÖĂąôĄąąőłężÉœűÒ»ČœžÉÔïNOĄąCl2Í⣏ÁíÒ»žöŚśÓĂÊÇ___________________ĄŁ
Łš3Ł©Ś°ÖĂÁŹËłĐòÎȘ aĄú_______________(°ŽÆűÁśŚÔŚóÏòÓÒ·œÏò,ÓĂĐĄĐŽŚÖÄž±íÊŸ)ĄŁ
Łš4Ł©Ś°ÖĂąő”ÄŚśÓĂÊÇ___________________________________ĄŁ
Łš5Ł©Ś°ÖĂąùÔÚÊ”ŃéʱŁŹÔ€ÆÚčÛČ씜”ÄÏÖÏóÊÇ__________________________________________ĄŁ
Łš6Ł©Ś°ÖĂąűÖĐÎüÊŐÎČÆűʱŁŹNOCl·ąÉú·ŽÓŠ”Ä»ŻŃ§·œłÌÊœÎȘ________________________ĄŁ
ĄŸÍŰŐčŃ§Ï°Ąż
Łš7Ł©ČéÔÄŚÊÁÏŁŹ”ĂÖȘĆäÖÆÍőËź(ĆšÏőËáÓëĆšŃÎËá”Ä»ìËá) ʱ»áÉúłÉŃÇÏőőŁÂÈșÍÂÈÆű,žĂ·ŽÓŠ”Ä»ŻŃ§·œłÌÊœÎȘ__________________________________ĄŁ