��Ŀ����
2��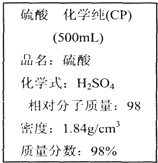 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴ˻ش�
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴ˻ش���1�����Լ������ʵ���Ũ��Ϊ18.4mol•L-1�ø���������100mL 4.6mol•L-1��ϡ����������Ͳ��ȡ������25.0mL����ȡŨ����ʱӦѡ�âڣ�ѡ����ţ���10mL����50mL����100mL��������Ͳ���ɹ�ѡ�õ������У��ٽ�ͷ�ι� ����ƿ ���ձ� ��ҩ�� ����Ͳ ��������ƽ������ϡ����ʱ�����������л�ȱ�ٵ�������100mL����ƿ����������д�������ƣ���
��2����ͭƬ���Ũ������ȷ�Ӧ������������ͨ��������ˮ�У�������Ӧ�����ӷ���ʽ��SO2+Cl2+2H2O=SO42-+2Cl-+4H+��
���� ��1������c=$\frac{1000�Ѧ�}{M}$�������Ũ��������ʵ���Ũ�ȣ��������ƹ��������ʵ����ʵ�������������ҪŨ�������������ݼ������ж���Ͳ���������һ�����ʵ���Ũ�ȵ���Һ�����ж�ʹ��������ȱ�ٵ�������
��2��ͭ��Ũ����������ɵ�����Ϊ����������������������Ӧ����������Ȼ��⣬�ݴ�д����Ӧ�����ӷ���ʽ��
��� �⣺��1����Ũ��������ʵ���Ũ��Ϊ��c=$\frac{1000�Ѧ�}{M}$=$\frac{1000��1.84��98%}{98}$mol/L=18.4mol/L��
�ø���������100mL 4.6mol•L-1��ϡ���ᣬ���ƹ�������������ʵ������䣬����Ҫ��Ũ��������Ϊ��$\frac{4.6mol/L��0.1L}{18.4mol/L}$=0.025L=25.0ml����ȡ25.0mLŨ������Ҫѡ�ù��Ϊ50mL����Ͳ������100mL��Һ��Ҫѡ��100mL������ƿ�����ƹ�������Ҫ�ò�������������������Ի�ȱ�ٵ�����Ϊ100mL����ƿ�Ͳ�������
�ʴ�Ϊ��18.4��25.0�� �ڣ�100mL����ƿ����������
��2����ͭƬ���Ũ������ȷ�Ӧ������������Ϊ����������������ͨ��������ˮ�У���Ӧ����������Ȼ��⣬��Ӧ�����ӷ���ʽ�ǣ�SO2+Cl2+2H2O=SO42-+2Cl-+4H+��
�ʴ�Ϊ��SO2+Cl2+2H2O=SO42-+2Cl-+4H+��
���� ���⿼�������ʵ�����Ũ�ȵļ��㡢���ӷ���ʽ��д������һ�����ʵ���Ũ�ȵ���Һ��������Ŀ�Ѷ��еȣ�ע���������ʵ���Ũ�ȵļ��㷽������ȷ���ӷ���ʽ��дԭ������һ�����ʵ���Ũ�ȵ���Һ�IJ��裮

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�| A�� | CH4 | B�� | C2H4 | C�� | C3H4 | D�� | C3H8 |
| A�� | AgCl��������AgBr���� | B�� | AgCl��AgBr������������ | ||
| C�� | AgCl��������AgBr���� | D�� | ֻ��AgBr�������� |
| A�� | ��С�մ���ҩ������θ����ࣺHCO3-+H+�TCO2��+H2O | |
| B�� | FeCl3��Һ��Cu�ķ�Ӧ��Cu+Fe3+�TCu2++Fe2+ | |
| C�� | ��Ba��OH��2��Һ�еμ�NaHSO4��Һ�������Һǡ��Ϊ���ԣ�Ba2++OH-+H++SO42-�TBaSO4��+H2O | |
| D�� | ������Һ��ˮ���е�CaCO3��Ӧ��CaCO3+2H+�TCa2++H2O+CO2�� |

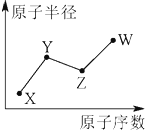
| A�� | X��Y��ZԪ�طֱ�ΪN��P��O | |
| B�� | ���ж�������Ԫ�أ��Ҹ��ж��Ǹ���Ԫ�� | |
| C�� | ԭ�Ӱ뾶��Z��X��Y | |
| D�� | X��Y��Z����̬�⻯�������ȶ����ǣ�Y���⻯�� |
| A�� | Fe3O4����������Fe2+�ǻ�ԭ�� | |
| B�� | ÿ����1mol NO����ת�Ƶ�����Ϊ2mol | |
| C�� | �μӷ�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ28��3 | |
| D�� | ����3mol Fe3O4����������ԭ��HNO3Ϊ1mol |
 ��
�� ҵ������M��ˮ��Ӧ�IJ���֮һ������Ư����ɱ����д��M��ˮ������Ӧ�Ļ�ѧ����ʽNCl3+3H2O�TNH3+3HClO��
ҵ������M��ˮ��Ӧ�IJ���֮һ������Ư����ɱ����д��M��ˮ������Ӧ�Ļ�ѧ����ʽNCl3+3H2O�TNH3+3HClO�� CH3COOCH2CH3+H2O���÷�Ӧ������Ϊȡ����Ӧ��
CH3COOCH2CH3+H2O���÷�Ӧ������Ϊȡ����Ӧ��