��Ŀ����
A��B��C��D��E��F�������ʵ��ת����ϵ����ͼ��ʾ(��Ӧ����δ���)�����з�Ӧ�����û���Ӧ��
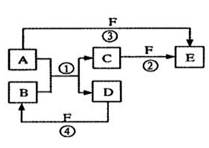
��1����A��D��F���Ƿǽ������ʣ���A��D����Ԫ��ͬ���壬A��F����Ԫ��ͬ���ڣ���Ӧ�ٵĻ�ѧ����ʽ��________ ___��
��2����A�dz����Ľ������ʣ�D��F����̬���ʣ���Ӧ�٢ھ���ˮ��Һ�н��еģ���E��ˮ��Һ�� �ԣ���ᡱ������С�������ԭ����(�����ӷ���ʽ��ʾ) ����֪1 g D��F��Ӧ����Bʱ�ų�92.3 kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ_____________ _____��
��3����B��C��F������̬���ʣ���B�ж����ۺ͢�������Ӧ�ж���ˮ���ɣ���Ӧ����Ҫ�ŵ���ܷ����� A��D�����а������ɣ� ��Ӧ�ٵĻ�ѧ����ʽ�� _______��
��4����A��DΪ������Ԫ�ص��ʣ�������Ԫ�ص�ԭ������A��D��2��������Ԫ�ص�ԭ�Ӻ�������������D��A��2�����ۺ͢�������Ӧ�ж��к���ɫ�������ɣ���Ӧ�ٻ�ѧ����ʽΪ______ _______ ��Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ _________________��

��1����A��D��F���Ƿǽ������ʣ���A��D����Ԫ��ͬ���壬A��F����Ԫ��ͬ���ڣ���Ӧ�ٵĻ�ѧ����ʽ��________ ___��
��2����A�dz����Ľ������ʣ�D��F����̬���ʣ���Ӧ�٢ھ���ˮ��Һ�н��еģ���E��ˮ��Һ�� �ԣ���ᡱ������С�������ԭ����(�����ӷ���ʽ��ʾ) ����֪1 g D��F��Ӧ����Bʱ�ų�92.3 kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ_____________ _____��
��3����B��C��F������̬���ʣ���B�ж����ۺ͢�������Ӧ�ж���ˮ���ɣ���Ӧ����Ҫ�ŵ���ܷ����� A��D�����а������ɣ� ��Ӧ�ٵĻ�ѧ����ʽ�� _______��
��4����A��DΪ������Ԫ�ص��ʣ�������Ԫ�ص�ԭ������A��D��2��������Ԫ�ص�ԭ�Ӻ�������������D��A��2�����ۺ͢�������Ӧ�ж��к���ɫ�������ɣ���Ӧ�ٻ�ѧ����ʽΪ______ _______ ��Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ _________________��
(1) 2C+SiO2  Si+2CO��
Si+2CO��
(2)�Fe3++ 3H2O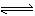 Fe(OH)3+3H+��H2(g) +Cl2(g)=2HCl(g)����H="-184.6" kJ/mol
Fe(OH)3+3H+��H2(g) +Cl2(g)=2HCl(g)����H="-184.6" kJ/mol
(3) 3Cl2+2NH3=6HCl+N2
(4)2Mg+CO2="C+2MgO " 2��1
 Si+2CO��
Si+2CO��(2)�Fe3++ 3H2O
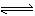 Fe(OH)3+3H+��H2(g) +Cl2(g)=2HCl(g)����H="-184.6" kJ/mol
Fe(OH)3+3H+��H2(g) +Cl2(g)=2HCl(g)����H="-184.6" kJ/mol(3) 3Cl2+2NH3=6HCl+N2
(4)2Mg+CO2="C+2MgO " 2��1
��

��ϰ��ϵ�д�
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�
�����Ŀ

 ���ӣ��������г�������˳���ǣ�����ţ� ��
���ӣ��������г�������˳���ǣ�����ţ� ��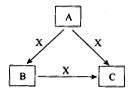
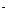 �ṹ��
�ṹ�� ��
��
 A�����ӷ���ʽ��_____________________________________
A�����ӷ���ʽ��_____________________________________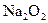 �μӷ�Ӧ�Ļ�ѧ����ʽ______________________________________��
�μӷ�Ӧ�Ļ�ѧ����ʽ______________________________________��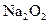 ת�Ƶĵ�����Ϊ_____________����
ת�Ƶĵ�����Ϊ_____________����
