��Ŀ����
��ʳ��Ϊԭ�Ͻ����������ۺ����õ�ijЩ��������ͼ��ʾ��

��1����ȥ�����е�Ca2+��Mg2+��SO ���ӣ��������г�������˳���ǣ�����ţ� ��
���ӣ��������г�������˳���ǣ�����ţ� ��
a��Na2CO3 b��NaOH c��BaCl2
��2������Һ��pH�������Գ�ȥ�������� ��
��3����ⱥ��ʳ��ˮ��Ӧ�����ӷ���ʽ�� ��
��4����������NaHCO3������ĸҺ�м��������ʯ�ң���ɻ��һ�ֿ���ѭ��ʹ�õ����ʣ��仯ѧʽ�� ��
��5�������������������й㷺��Ӧ�á�
�ٴ�������ڳ���̨���ۡ���ԭ���ǣ�������ӷ���ʽ������ ��
�ڳ����£���ijpH=11��Na2CO3��Һ�м������ʯ���飬���˺�������ҺpH=13��
��Ӧǰ����Һ���뷴Ӧ�����Һ��ˮ�������c(OH��)�ı�ֵ�� ��
�۹�ҵ�ϣ������ô�������ռ�����ijЩ������Ʒ�����ñ��ʹ�����Һ��Cl2��Ӧ��ȡ��Ч�ɷ�ΪNaClO������Һ���䷴Ӧ�����ӷ���ʽ�� ������֪̼�������ǿ�ڴ����ᣩ��

��1����ȥ�����е�Ca2+��Mg2+��SO
 ���ӣ��������г�������˳���ǣ�����ţ� ��
���ӣ��������г�������˳���ǣ�����ţ� ��a��Na2CO3 b��NaOH c��BaCl2
��2������Һ��pH�������Գ�ȥ�������� ��
��3����ⱥ��ʳ��ˮ��Ӧ�����ӷ���ʽ�� ��
��4����������NaHCO3������ĸҺ�м��������ʯ�ң���ɻ��һ�ֿ���ѭ��ʹ�õ����ʣ��仯ѧʽ�� ��
��5�������������������й㷺��Ӧ�á�
�ٴ�������ڳ���̨���ۡ���ԭ���ǣ�������ӷ���ʽ������ ��
�ڳ����£���ijpH=11��Na2CO3��Һ�м������ʯ���飬���˺�������ҺpH=13��
��Ӧǰ����Һ���뷴Ӧ�����Һ��ˮ�������c(OH��)�ı�ֵ�� ��
�۹�ҵ�ϣ������ô�������ռ�����ijЩ������Ʒ�����ñ��ʹ�����Һ��Cl2��Ӧ��ȡ��Ч�ɷ�ΪNaClO������Һ���䷴Ӧ�����ӷ���ʽ�� ������֪̼�������ǿ�ڴ����ᣩ��
��1����1�֣�c a b ���� c b a����b c a
��2����1�֣�CO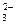 ��OH��
��OH��
��3����2�֣�2Cl��+ 2H2O 2OH��+ H2�� + Cl2��
2OH��+ H2�� + Cl2��
��4����2�֣�NH3
��5���٣�2�֣�CO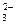 ˮ���Լ���CO
ˮ���Լ���CO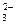 + H2O
+ H2O HCO
HCO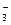 +OH���������ڼ���������ˮ�⣬�ﵽȥ��Ŀ�ġ�
+OH���������ڼ���������ˮ�⣬�ﵽȥ��Ŀ�ġ�
�ڣ�2�֣�1��1010
�ۣ�2�֣�2CO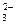 + Cl2 + H2O �� Cl��+ ClO��+ 2HCO
+ Cl2 + H2O �� Cl��+ ClO��+ 2HCO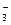
��2����1�֣�CO
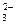 ��OH��
��OH����3����2�֣�2Cl��+ 2H2O
 2OH��+ H2�� + Cl2��
2OH��+ H2�� + Cl2����4����2�֣�NH3
��5���٣�2�֣�CO
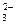 ˮ���Լ���CO
ˮ���Լ���CO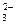 + H2O
+ H2O HCO
HCO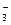 +OH���������ڼ���������ˮ�⣬�ﵽȥ��Ŀ�ġ�
+OH���������ڼ���������ˮ�⣬�ﵽȥ��Ŀ�ġ��ڣ�2�֣�1��1010
�ۣ�2�֣�2CO
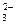 + Cl2 + H2O �� Cl��+ ClO��+ 2HCO
+ Cl2 + H2O �� Cl��+ ClO��+ 2HCO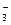
��

��ϰ��ϵ�д�
 ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д� ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
�����Ŀ
 ����ѧ����ʽ��
����ѧ����ʽ�� 
 ��
��

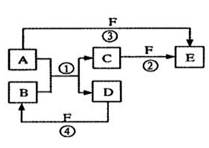
 д����Eת���F������ ���䷢����Ӧ�Ļ�ѧ����ʽ________��
д����Eת���F������ ���䷢����Ӧ�Ļ�ѧ����ʽ________��