��Ŀ����
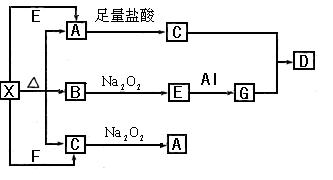
(14��)��ͼ��ʾ�ķ�Ӧ��ϵ�У����ֲ��ﱻ��ȥ����֪2mol��ɫ�����ĩ���ȷֽ⣬�ָ����������ɰ�ɫ����A.��ɫҺ��B.��ɫ����C��1mol��X.E.G����ɫ��Ӧ��Ϊ��ɫ��
�ش��������⣺
(1)д���������ʵĻ�ѧʽ��G_____________ D_____________
(2)д��G��C��Ӧ����D�Ļ�ѧ��Ӧ����ʽ��_________________________________
(3)д��X��E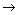 A�����ӷ���ʽ��_____________________________________
A�����ӷ���ʽ��_____________________________________
(4)д��C��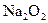 �μӷ�Ӧ�Ļ�ѧ����ʽ______________________________________��
�μӷ�Ӧ�Ļ�ѧ����ʽ______________________________________��
��0.2mol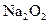 ת�Ƶĵ�����Ϊ_____________����
ת�Ƶĵ�����Ϊ_____________����
(5)д������X����;������д��һ�֣�______________________________________

�ش��������⣺
(1)д���������ʵĻ�ѧʽ��G_____________ D_____________
(2)д��G��C��Ӧ����D�Ļ�ѧ��Ӧ����ʽ��_________________________________
(3)�X��E
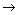 A�����ӷ���ʽ��_____________________________________
A�����ӷ���ʽ��_____________________________________(4)д��C��
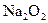 �μӷ�Ӧ�Ļ�ѧ����ʽ______________________________________��
�μӷ�Ӧ�Ļ�ѧ����ʽ______________________________________����0.2mol
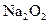 ת�Ƶĵ�����Ϊ_____________����
ת�Ƶĵ�����Ϊ_____________����(5)д������X����;������д��һ�֣�______________________________________
��14�֣�
�� NaAlO2 Al(OH)3
�� NaAlO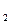 +CO2+2H2O=Al(OH) 3��+NaHCO3��2NaAlO
+CO2+2H2O=Al(OH) 3��+NaHCO3��2NaAlO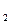 +CO2+3H2O=2Al(OH) 3��+Na2CO3
+CO2+3H2O=2Al(OH) 3��+Na2CO3
�� HCO3��+OH��=CO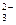 +H2O
+H2O
�� 2CO2+2Na2O2=2Na2CO3+O2 0.2��6.02��10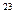
(5) ���ͷۡ�����θ������
�� NaAlO2 Al(OH)3
�� NaAlO
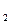 +CO2+2H2O=Al(OH) 3��+NaHCO3��2NaAlO
+CO2+2H2O=Al(OH) 3��+NaHCO3��2NaAlO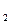 +CO2+3H2O=2Al(OH) 3��+Na2CO3
+CO2+3H2O=2Al(OH) 3��+Na2CO3�� HCO3��+OH��=CO
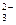 +H2O
+H2O�� 2CO2+2Na2O2=2Na2CO3+O2 0.2��6.02��10
(5) ���ͷۡ�����θ������
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 д����Eת���F������ ���䷢����Ӧ�Ļ�ѧ����ʽ________��
д����Eת���F������ ���䷢����Ӧ�Ļ�ѧ����ʽ________��