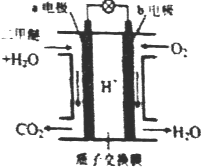
��Ŀ����
����Ŀ��������ⷨ���Ѱ۲����ķ�Һ[���д���FeSO4��H2SO4������Fe2(SO4)3��TiOSO4]��������Ͳ�Ѫ�������������������������£�
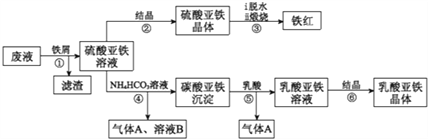
��֪:TiOSO4������ˮ����ˮ�п��Ե���ΪTIO2+��SO42-��TiOSO4ˮ���TiO2��xH2O����Ϊ���淴Ӧ������ṹ��ʽΪCH3CH(OHCOOH.��ش�
(1)������з�������������Һ�������IJ�����______________��
(2)������м��Ŀһ�ǻ�ԭ����Fe2(SO4)3������ʹ����TiOSO4ת��ΪTiO2��xH2O��������ƽ���ƶ���ԭ�����͵õ�������ԭ��_______________��
(3)���������ڿ�������������������������÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ_____________��
(4)�����ӷ���ʽ���Ͳ�����м������ܵõ�����������ԭ��___________________��
(5)����ܵ����ӷ���ʽ��________________��
(6)����ޱ������һ������նȣ�ԭ��������������ˮ�Լ�_______________��
(7)������������{[CH3CH(OH)COO]2Fe��3H2O}���ȵIJ���������KMnO4�ζ����ⶨ��Ʒ��Fe�����������㴿��ʱ�����ֽ�����Ǵ���100%����ԭ�������_______________��
������������Ce(SO4)2����Һ�ζ����вⶨ����Ӧ��Ce4+���ӵĻ�ԭ����ΪCe3+���ⶨʱ���ȳ�ȡ5.760g��Ʒ���ܽ����б�Ҫ������������ƿ���Ƴ�250mL��Һ��ÿ��ȡ25.00mL����0.1000mol/LCe(SO4)2����Һ�ζ����յ��¼�������±���
�ζ����� | 0.1000mol/LCe(SO4)2����Һ/mL | |
�ζ�ǰ���� | �ζ������ | |
1 | 0.10 | 19.85 |
2 | 0.12 | 21.32 |
3 | 1.05 | 20.70 |
4 | 0.16 | 19.88 |
���Ʒ��������������Ĵ���Ϊ_______(������������ʾ)��
���𰸡� ���� TiOSO4+(x+1)H2O![]() TiO2 xH2O��+H2SO4��TiO2++(x+1)H2O
TiO2 xH2O��+H2SO4��TiO2++(x+1)H2O![]() TiO2 xH2O��+2H+��м��H2SO4��Ӧ��c(H+)���ͣ�ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O���� 1: 4 FeCO3+2CH3CH(OH)COOH== Fe2++2CH3CH(OH)COO-+H2O+CO2�� Fe2++2HCO3-==FeCO3��+H2O+CO2�� ��ֹFe2+������ ��������ǻ�(��OH)�����Ը��������Һ���� 98.5%�� 0.985
TiO2 xH2O��+2H+��м��H2SO4��Ӧ��c(H+)���ͣ�ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O���� 1: 4 FeCO3+2CH3CH(OH)COOH== Fe2++2CH3CH(OH)COO-+H2O+CO2�� Fe2++2HCO3-==FeCO3��+H2O+CO2�� ��ֹFe2+������ ��������ǻ�(��OH)�����Ը��������Һ���� 98.5%�� 0.985
�������������������1��ʵ�ֹ����Һ��ķ����ù��˵ķ�����(2) TiO2++(x+1)H2O![]() TiO2 xH2O��+2H+����м��H2SO4��Ӧ��c(H+)������(3)���������ڿ��������������������������ķ���ʽΪ��4FeSO4+O2
TiO2 xH2O��+2H+����м��H2SO4��Ӧ��c(H+)������(3)���������ڿ��������������������������ķ���ʽΪ��4FeSO4+O2![]() 2Fe2O3+4SO3������������������ԭ������������(4) ̼���������������ܽ�ƽ�⣺FeCO3��s��Fe2+��aq��+CO32-��aq�����������ᣬCO32-�����ᷴӦŨ�Ƚ��ͣ�ƽ�������ƶ���ʹ̼�������ܽ�õ�����������Һ��(5)������ͼ��֪������������̼����立�Ӧ����̼������������������Ϊ������̼��(6)���������ױ��������������Բ���ޱ������һ������նȣ�(7) [CH3CH(OH)COO]2Fe��3H2O�е�������������������ǻ�(��OH)���ܱ����Ը��������Һ���������ݱ������ݣ��ڶ��βⶨ����ƫ��������Χ�����õ�һ�����������Ĵ�ʵ�����ݣ�ƽ������0.1000mol/LCe(SO4)2����Һ19.70mL�����ݹ�ϵʽ
2Fe2O3+4SO3������������������ԭ������������(4) ̼���������������ܽ�ƽ�⣺FeCO3��s��Fe2+��aq��+CO32-��aq�����������ᣬCO32-�����ᷴӦŨ�Ƚ��ͣ�ƽ�������ƶ���ʹ̼�������ܽ�õ�����������Һ��(5)������ͼ��֪������������̼����立�Ӧ����̼������������������Ϊ������̼��(6)���������ױ��������������Բ���ޱ������һ������նȣ�(7) [CH3CH(OH)COO]2Fe��3H2O�е�������������������ǻ�(��OH)���ܱ����Ը��������Һ���������ݱ������ݣ��ڶ��βⶨ����ƫ��������Χ�����õ�һ�����������Ĵ�ʵ�����ݣ�ƽ������0.1000mol/LCe(SO4)2����Һ19.70mL�����ݹ�ϵʽ![]() ���㴿����
���㴿����
��������1��ʵ�ֹ����Һ��ķ����ù��˵ķ�����(2) TiO2++(x+1)H2O![]() TiO2 xH2O��+2H+����м��H2SO4��Ӧ��c(H+)������ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O������(3)���������ڿ��������������������������ķ���ʽΪ��4FeSO4+O2
TiO2 xH2O��+2H+����м��H2SO4��Ӧ��c(H+)������ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O������(3)���������ڿ��������������������������ķ���ʽΪ��4FeSO4+O2![]() 2Fe2O3+4SO3������������������ԭ�����������������������ͻ�ԭ�������ʵ���֮��Ϊ1��4��(4) ̼���������������ܽ�ƽ�⣺FeCO3��s��Fe2+��aq��+CO32-��aq�����������ᣬCO32-�����ᷴӦŨ�Ƚ��ͣ�ƽ�������ƶ���ʹ̼�������ܽ�õ�����������Һ����Ӧ����ʽ��FeCO3+2CH3CH(OH)COOH== Fe2++2CH3CH(OH)COO-+H2O+CO2����(5)������ͼ��֪������������̼����立�Ӧ����̼������������������Ϊ������̼����Ӧ���ӷ���ʽ��Fe2++2HCO3-==FeCO3��+H2O+CO2����(6)���������ױ��������������Բ���ޱ������һ������նȣ����Կ���һ������ն�����������ˮ���ܷ�ֹFe2+��������(7) [CH3CH(OH)COO]2Fe��3H2O�е�������������������ǻ�(��OH)���ܱ����Ը��������Һ���������Խ�����Ǵ���100%����25mL��Һ����CH3CH(OH)COO]2Fe��3H2O�����ʵ���xg��
2Fe2O3+4SO3������������������ԭ�����������������������ͻ�ԭ�������ʵ���֮��Ϊ1��4��(4) ̼���������������ܽ�ƽ�⣺FeCO3��s��Fe2+��aq��+CO32-��aq�����������ᣬCO32-�����ᷴӦŨ�Ƚ��ͣ�ƽ�������ƶ���ʹ̼�������ܽ�õ�����������Һ����Ӧ����ʽ��FeCO3+2CH3CH(OH)COOH== Fe2++2CH3CH(OH)COO-+H2O+CO2����(5)������ͼ��֪������������̼����立�Ӧ����̼������������������Ϊ������̼����Ӧ���ӷ���ʽ��Fe2++2HCO3-==FeCO3��+H2O+CO2����(6)���������ױ��������������Բ���ޱ������һ������նȣ����Կ���һ������ն�����������ˮ���ܷ�ֹFe2+��������(7) [CH3CH(OH)COO]2Fe��3H2O�е�������������������ǻ�(��OH)���ܱ����Ը��������Һ���������Խ�����Ǵ���100%����25mL��Һ����CH3CH(OH)COO]2Fe��3H2O�����ʵ���xg��
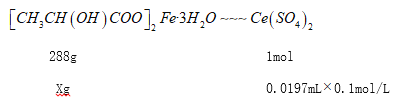
![]()
X=0.56736g����Ʒ��������������Ĵ���Ϊ![]() ��
��

 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�����Ŀ�����Ӻ�Br-�ķ�Һ����ȡ�嵥�ʣ��辭��һϵ�в�����ʵ��װ�ü����ʵ�����������

Br2 | CCl4 | ��ʮ���� | |
�ܶ�/g��cm-3 | 3. 12 | 1.59 | 0.753 |
�е�/�� | 58.76 | 76.8 | 215��217 |
����˵������ȷ����
A. ����װ�ü�������Һ�е�Br-
B. װ������ѡ����ʮ���������CCl4������Ϊ��ʮ������ܶȸ�С
C. ��װ�ñ������������ռ���ʮ�������ռ�Br2
D. ��װ�ö���������Һ��
����Ŀ�������£��������ʵ��ܶȻ��������±���
���� | Cu(OH)2 | Fe(OH)3 | CuCl | CuI |
Ksp | 2.2��10��20 | 2.6��10��39 | 1.7��10��7 | 1.3��10��12 |
��1��ij����CuCl2��Һ�к�������FeCl3��Ϊ�Ƶô���CuCl2��Һ���˼���__________������Һ����pH��4��ʹFe3��ת��ΪFe(OH)3��������ʱ��Һ�е�c(Fe3��)��____________________ mol��L��1��
��2��������Һ���˺�������Һ����________��________(������˳����дʵ�鲽�������)���پ����ˣ��ɵõ�CuCl2��2H2O���塣
��3��ijѧϰС���á���ӵ��������ⶨ����CuCl2��2H2O���������(��������I��������Ӧ������������)�Ĵ��ȣ��������£�ȡ0.800 g��������ˮ�������ʵ�������KI���壬��ַ�Ӧ�����ɰ�ɫ��������0.100 0 mol��L��1Na2S2O3����Һ�ζ�������ζ��յ�ʱ������Na2S2O3����Һ40.00 mL(��֪��I2��2S2O![]() ===S4O
===S4O![]() ��2I�� )��
��2I�� )��
�ٿ�ѡ��________���ζ�ָʾ�����ζ��յ��������______________________��
��CuCl2��Һ��KI��Ӧ�����ӷ���ʽΪ________________________________��
�ۺ���CuCl2��2H2O����������Ĵ�����__________________��