题目内容
【题目】一定条件下,在体积为3 L的密闭容器中,一氧化碳与氢气反应生成甲醇(催化剂为Cu2O/ZnO):CO(g)+2H2(g)CH3OH(g)。
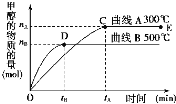
根据题意完成下列各题:
(1)反应达到平衡时,平衡常数表达式K=__________,升高温度,K值__________(填“增大”、“减小”或“不变”)。
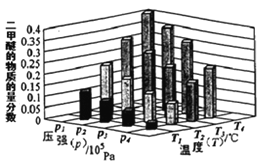
(2)在500℃,从反应开始到平衡,氢气的平均反应速率v(H2)=______________。
(3)在其他条件不变的情况下,对处于E点的体系体积压缩到原来的1/2,下列有关该体系的说法正确的是__________(填字母序号)。
a.氢气的浓度减小 b.正反应速率加快,逆反应速率也加快
c.甲醇的物质的量增加 d.重新平衡时n(H2)/n(CH3OH)增大
【答案】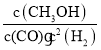 减小
减小 ![]() mol·L-1·min-1 bc
mol·L-1·min-1 bc
【解析】
(1)根据方程式和K的含义书写;根据温度对平衡的影响,判断K的变化;
(2)根据图中甲醇的变化量求出氢气的变化量,再根据![]() 计算;
计算;
(3)在其他条件不变的情况下,对处于E点的体系体积压缩到原来的1/2,则压强增大,正逆反应速率都增大,平衡向正向移动,甲醇的物质的量增多,氢气的物质的量减小,但因为体积减小,平衡时氢气的浓度反而增大,根据浓度比值等于物质的量比值。
(1)已知CO(g)+2H2(g)CH3OH(g),则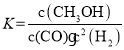 ,500℃时甲醇的物质的量小,所以升高温度,平衡逆移,所以平衡常数K减小,故答案为:
,500℃时甲醇的物质的量小,所以升高温度,平衡逆移,所以平衡常数K减小,故答案为: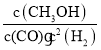 ;减小;
;减小;
(2)在500℃,平衡时图中甲醇的变化量为nB,所以反应消耗氢气的量为2nB,则![]() ,故答案为:
,故答案为:![]() mol·L-1·min-1;
mol·L-1·min-1;
(3) 在其他条件不变的情况下,对处于E点的体系体积压缩到原来的1/2,则压强增大,正逆反应速率都增大,平衡向正向移动,甲醇的物质的量增多,氢气的物质的量减小,但因为体积减小,平衡时氢气的浓度反而增大,根据浓度比值等于物质的量比值,则有重新平衡时![]() 减小,即bc正确,故答案为bc。
减小,即bc正确,故答案为bc。