��Ŀ����
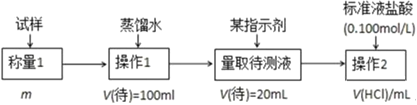
����Ŀ�������£���pH�������������ֻ�ʵ�飬�ֱ�������ʢ50mL0.100mol/L������ձ������ٵμ�50mLȥ����ˮ��50mL0.100mol/L �������Һ���μӹ��̽��д������裬�����ҺpH��ʱ��仯��ͼ��ʾ����֪�����´������ҺpH��7������˵���������
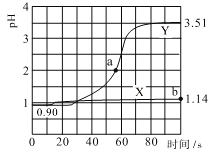
A.����X��ʾ�����мӴ������Һϡ�͵�pH�仯
B.����Y��pH�仯��Ҫ����ΪCH3COO-��H+��ϳ����������
C.a���Ӧ����Һ��c(Cl-)��c(CH3COO-)��c(CH3COOH)��c(NH4+)��0.01mol/L
D.b���Ӧ����Һ��ˮ�����c(H+)��10-12.86 mol/L
���𰸡�AC
��������
�ֱ�������ʢ50mL0.100mol/L������ձ������ٵμ�50mLȥ����ˮ��50mL0.100mol/L�������Һ����ˮϡ�Ͷ�pH�仯Ӱ���С��������������ӦH++CH3COO-CH3COOH�������������CH3COOH����Һ��������Ũ��Ѹ�ټ��٣�pH�仯�ϴ���X���߱�ʾ�����м�ˮϡ�ͣ�����Y��ʾ�����м�����ᣬ�ݴ˽�ϵ���غ������
A������X�仯����ʾ�����м�ˮϡ�͵�pH�仯����A����
B������Y�仯�ϴ�˵��������ӦH++CH3COO-CH3COOH��CH3COO-��H+��ϳ���������ʣ���Һ��������Ũ��Ѹ�ټ��٣���B��ȷ��
C��a��pH=2��c(H+)=0.01mol/L�����ݵ���غ��֪��c(OH-)+c(Cl-)+c(CH3COO-)=c(H+)+c(NH4+)=c(NH4+)+0.01mol/L����c(OH-)+c(Cl-)+c(CH3COO-)-c(NH4+)=0.01mol/L����C����
D������ͼʾ��֪��b����Һ��pH=1.14��c(H+)=10-1.14mol/L��������������ˮ�ĵ��룬��Һ����������������ˮ�ĵ��룬��ˮ�����c(H+)=c(OH-)=10-12.86mol/L����D��ȷ��
��ѡAC��

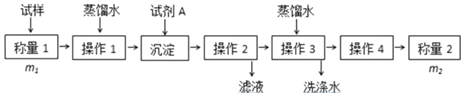
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�����Ŀ����������ۺ����ü������ڽ�Լ��Դ���������ڱ���������ij�����Թ�ҵ������Cr��III�������������ù���������ͼ�������ȡҺ�н���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+����

��֪�������£�����������������������ʽ����ʱ��Һ��pH����ʼ������pH������������Ũ��Ϊ1.0mol��L-1���㣩����ȫ����ʱ��Һ��pH�����±���
������ | Fe3+ | Fe2+ | Mg2+ | Al3+ | Cu2+ | Cr3+ |
��ʼ����ʱ��pH | 1.9 | 7.5 | ���� | ���� | 4.7 | ���� |
������ȫʱ��pH | 3.2 | 9.7 | 11.1 | 8 | 6.7 | 9����9�ܽ⣩ |
�ظ�������ӣ�Cr2O72-������Һ�д�������ƽ�⣺2CrO42-+2H+![]() Cr2O72-+H2O
Cr2O72-+H2O
��1���ù����Թ�ҵ������Cr��+3�ۣ������������ù��������У�����һ���������������ij����ܽ�ƽ�⣺Fe(OH)3(s)![]() Fe3+(aq)+3OH-(aq)�����ܶȻ���������ʽΪKsp=__��
Fe3+(aq)+3OH-(aq)�����ܶȻ���������ʽΪKsp=__��
��2�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ��__�����ٴ�һ�㣩��
��3������H2O2��������__��������Һ��pH=8��Ϊ�˳�ȥ__���ӡ�
��4�������ӽ�����֬��ԭ��Ϊ��Mn++nNaR��MRn+nNa+���������ĵ�����������__��