��Ŀ����
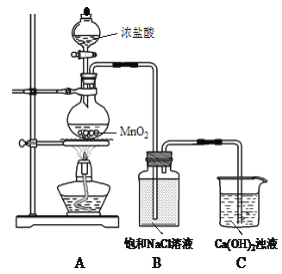
����Ŀ���̷���FeSO4��7H2O�����IJⶨ�������������ⶨ�̷���FeSO4��7H2O�ĺ������ζ���Ӧ�ǣ�5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
ʵ�鷽�����£��ٳ�ȡ15.041g�̷���Ʒ���ܽ⣬��250mL����ƿ�ж��ݡ�
����ȡ25.00mL ������Һ����ƿ�С�
����0.0500mol/LKMnO4��Һ�������ữ���ζ����յ㣬��¼��������ĩ������
�� ��
�� ���ݴ�����
��1������ʵ�鷽���еIJ������______���ྻ�ĵζ����ڵζ�ǰ������еIJ����У�
�ټ�������Ƿ�©ˮ��
��_______��
����ζ����м���KMnO4����Һ��0�̶����ϣ������첿λ�����ݣ����ڳ�������
�����ݴ�����ijС��ͬѧ��ʵ�����ݼ�¼���£�
ʵ����� | ��������mL�� | ĩ������mL�� |
1 | 0.20 | 21.40 |
2 | 0.00 | 21.00 |
3 | 1.60 | 26.60 |
��2��������Ʒ��FeSO4��7H2O����������Ϊ_______����С����ʾ��������λС������
���𰸡��ظ������ڢ�1��2�� ������ˮϴ��2��3�Σ����ø�����ر���Һ��ϴ�ζ���2��3�� 0.975
��������
��1��Ϊ����ʵ�������ظ��ڢ�12�Σ�����ƽ��ʵ��12�Σ��ྻ�ĵζ����ڵζ�ǰ������м�©����ϴ����������ˮϴ��23�Σ����ø�����ر���Һ��ϴ�ζ���23�Σ�
�ʴ�Ϊ���ظ������ڢ�12�Σ�������ˮϴ��23�Σ����ø�����ر���Һ��ϴ�ζ���23�Σ�
��2���������ữ��0.01000mol/LKMnO4��Һ�ζ����յ㣬����KMnO4��Һ����������е������������ϴ���ȥ�����KMnO4��Һ�����ƽ��ֵΪ![]() =20.10mL����:
=20.10mL����:
5Fe2++MnO4+8H+�T5Fe3++Mn2++4H2O
51
n(Fe2+) 0.05000mol/L��0.0210L
����õ���n(Fe2+)=0.05000mol/L��0.0210L��5 =0.00525 mol��
��250mL��Һ�к�Fe2+=0.00525 mol��![]() =0.0525 mol��
=0.0525 mol��
FeSO47H2O���ʵ���Ϊ0.0525mol������=0.0525 mol��278g/mol=14.595 g��
��������=![]() ��100%=97.5%=0.975��
��100%=97.5%=0.975��
�ʴ�Ϊ��0.975��

����Ŀ������ˮ�ܿ���Ҫ�ɷ�ΪCo2O3��������Fe2O3��Al2O3��MnO��MgO��CaO��SiO2�ȣ�������ȡ���ֻ����Լ�������Ϊ�����ܾ�����Ȼ��ܾ�����Ʊ����̣��ش��������⣺
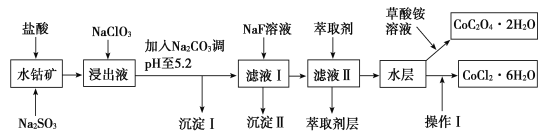
��֪���ٽ���Һ�к��е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Al3+��Mg2+��Ca2+�ȡ�
�ڳ�������ֻ�������ֳ�����
�������в���������������������ʽ����ʱ��Һ��pH������
������ | ��ʼ���� | ��ȫ���� |
Fe(OH)3 | 2.7 | 3.7 |
Fe(OH)2 | 7.6 | 9.6 |
Co(OH)2 | 7.6 | 9.2 |
Al(OH)3 | 4.0 | 5.2 |
Mn(OH)2 | 7.7 | 9.8 |
��1�������������������뻹ԭ�����ʵ���֮��Ϊ___��
��2��NaClO3�ڽ���Һ�з�����Ӧ�����ӷ���ʽΪ___��
��3������Na2CO3��pH��5.2��Ŀ����___����ȡ���㺬��Ԫ�أ�����������Ҫ�ɷ�Ϊ__��
��4���������������ˮ�����Ũ�������pHΪ2��3��___��___�����ˡ�ϴ�ӡ���ѹ��ɵȹ��̡�
��5��Ϊ�ⶨ�ֲ�Ʒ��CoCl2��6H2O�ĺ�������ȡһ�������Ĵֲ�Ʒ����ˮ���������������ữ����������Һ�����ˡ�ϴ�ӡ���������������ͨ�����㷢�ֲִ�Ʒ��CoCl2��6H2O������������100%����ԭ�������___���ش�һ��ԭ�ɣ���
��6����5.49g�����ܾ���(CoC2O4��2H2O)���ڿ����м��ȣ����ȹ����в�ͬ�¶ȷ�Χ�ڷֱ�õ�һ�ֹ������ʣ������������
�¶ȷ�Χ/�� | 150��210 | 290��320 |
��������/g | 4.41 | 2.41 |
���ⶨ���������ȹ��̣�ֻ����ˮ������CO2���壬��290��320���¶ȷ�Χ��ʣ��Ĺ������ʻ�ѧʽΪ___��[��֪��CoC2O4��2H2O��Ħ������Ϊ183g��mol-1]
����Ŀ����һ���2L���ܱ������м��뷴Ӧ��N2��H2���������·�Ӧ��N2(g)��3H2(g)![]() 2NH3(g)����Ӧ�����еIJ����������±���ʾ������˵����ȷ����
2NH3(g)����Ӧ�����еIJ����������±���ʾ������˵����ȷ����
���ʵ���/ mol ʱ��/min | n(N2) | n(H2) | n(NH3) |
0 | 1.0 | 1.2 | 0 |
2 | 0.9 | ||
4 | 0.75 | ||
6 | 0.3 |
A. 0��2 min�ڣ�NH3�ķ�Ӧ����Ϊ0.1 mol��L��1��min��1
B. 2 minʱ�� H2�����ʵ���0.3 mol
C. 4 minʱ����Ӧ�Ѵﵽƽ��״̬����ʱ�����淴Ӧ�����ʶ�Ϊ0
D. 4��6 min�ڣ�������������ӵ������ʵ�������