��Ŀ����
����Ŀ����������Ϊ����Խ�����ܱ���Ϊ��δ�����������乤ҵұ���漰���ķ�Ӧ������TiO2+2C+2Cl2![]() TiCl4+2CO
TiCl4+2CO
�ش��������������
��1���ѵļ۲�����Ų�ʽΪ______________��
��2����֪���������۷е�������
���� | ���ʯ | ���ʯ | ���Ȼ��� | ���廯�� | �ĵ⻯�� |
��ѧʽ | TiO2 | C | TiCl4 | TiBr4 | TiI4 |
���� | 1830�� | 3550�� | -24.1�� | 38�� | 150�� |
�е� | 2927�� | 4827�� | 136.4�� | 233.1�� | 377.2�� |
���� | ���Ӿ��� | ���Ӿ��� | |||
���н��ʯ�ľ���Ϊ_________���������������е������ѵ�±�����۷е��������ߵ�ԭ����_____________��
��3����λ��Ϊ6�����ΪTiCl3��6H2O�ľ�������������ѧʽΪ[TiCl(H2O)5]Cl2��H2O��X����ɫ������ʵ�������X��AgNO3��1��2���ʵ����ȷ�Ӧ���ɳ�����Y����ɫ����Y��AgNO3��1��3���ʵ����ȷ�Ӧ���ɳ�������Y�Ļ�ѧʽΪ___________��Y�����ӵĿռ乹��Ϊ_________��
��4�����ѿ�����Ҫ�ĺ��ѿ���֮һ������Ҫ�ɷ�Z�ľ�������ͼ��ʾ��
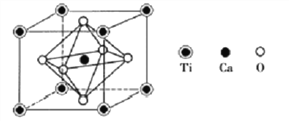
�Ʋ�Z�Ļ�ѧʽΪ________________��
��5������������a=384.1pm��Z������ܶ�Ϊ______g/cm3(��֪NA��6.0��1023��3.8413��![]() )��
)��
���𰸡� 3d24s2 ԭ�Ӿ��� ���Ӿ�����ɺͽṹ����ʱ����Է�������Խ���Ӽ�������Խ�����۷е�Խ�� [Ti(H2O)6]Cl3 �ռ��������� CaTiO3 4.0
�������������������1����ԭ�Ӻ�����22�����ӣ��۵����Ų���3d��4s�������2�����ݽ��ʯ���۵�߷��������Ӿ�����ɺͽṹ����ʱ����Է�������Խ���Ӽ�������Խ�����۷е�Խ������3��Y��AgNO3��1��3���ʵ����ȷ�Ӧ���ɳ�����˵�������3�������ӣ�6��ˮ�����ڽ磻��4�����ݾ�̯ԭ�����Z�Ļ�ѧʽ����5�����ݻ�ѧʽ��֪Z��Ħ��������136g/mol�������������![]() ��
��
��������1����ԭ�Ӻ�����22�����ӣ��۵����Ų���3d��4s������۵����Ų�Ϊ3d24s2����2�����ʯ���۵�������Խ��ʯ��ԭ�Ӿ����� TiCl4��TiBr4��TiI4���Ƿ��Ӿ��������Ӿ�����ɺͽṹ����ʱ����Է�������Խ���Ӽ�������Խ�����۷е�Խ�ߣ�����TiCl4��TiBr4��TiI4���۷е�������������3��Y��AgNO3��1��3���ʵ����ȷ�Ӧ���ɳ�����˵�������3�������ӣ�6��ˮ�����ڽ磬Y�Ļ�ѧʽΪ[Ti(H2O)6]Cl3��Y�����ӵĿռ乹��Ϊ�ռ�������������4�����ݾ�̯ԭ����ÿ����������Tiԭ������![]() =1������Caԭ����1������Oԭ������
=1������Caԭ����1������Oԭ������![]() ����Z�Ļ�ѧʽ��CaTiO3����5�����ݻ�ѧʽ��֪Z��Ħ��������136g/mol�������������
����Z�Ļ�ѧʽ��CaTiO3����5�����ݻ�ѧʽ��֪Z��Ħ��������136g/mol�������������![]() ����
����![]() g/cm3��
g/cm3��

 һ����������ϵ�д�
һ����������ϵ�д�
