��Ŀ����
����Ŀ�������廯����A(����R��δ֪����)��һ�������¿�ת��Ϊ����ϵ�����ʡ�
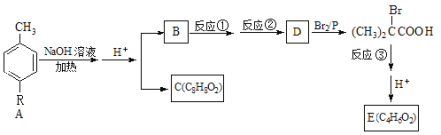
��֪��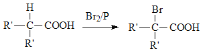 (R��Ϊ���)
(R�����)
�ش��������⣺
(1)A�Ľṹ��ʽ___________________________��B�Ľṹ��ʽ____________________��
(2)д����������������C��һ��ͬ���칹��Ľṹ��ʽ_______________________��
(I)�������ࣻ(II)������ֻ��һ��ȡ������(III)�ܷ���������Ӧ��
(3)���鷴Ӧ���Ƿ���ȫ�ķ�����_______________________________________________��
(4)��Ӧ�ٷ�Ӧ������______________________________��
(5)д����Ӧ�۵Ļ�ѧ����ʽ___________________________________________________��
(6) E���Ժϳɸ߾��д���ø߾���Ľṹ��ʽ__________________________________��
���𰸡� (CH3)2CHCH2OH
(CH3)2CHCH2OH ![]() ȡ��������������������������Һ�к��ᣬ�ټ�������������ͭ����У�������ש��ɫ������˵����Ӧδ��ȫ ������Ӧ
ȡ��������������������������Һ�к��ᣬ�ټ�������������ͭ����У�������ש��ɫ������˵����Ӧδ��ȫ ������Ӧ  +2NaOH
+2NaOH +NaBr+2H2O
+NaBr+2H2O 
��������
����A����B��C�ķ�Ӧ��������֪A��������ˮ�ⷴӦ�����κʹ����ٸ���ǿ��������ԭ����֪B��CΪ�������ᣬ����C�ķ���ʽ��ȷ��CΪ���� ��BΪ���������м����
��BΪ���������м���� ����֪B�������������������ᣬȷ��BΪ (CH3)2CHCH2OH��B������������D��(CH3)2CHCOOH����D��Br2����ȡ����Ӧ������
����֪B�������������������ᣬȷ��BΪ (CH3)2CHCH2OH��B������������D��(CH3)2CHCOOH����D��Br2����ȡ����Ӧ������ ������E�ķ���ʽ��֪
������E�ķ���ʽ��֪ ������ȥ��Ӧ����E��CH2=C��CH3��COOH��������B��C�Ľṹ��ʽ���Ƴ�AΪ
������ȥ��Ӧ����E��CH2=C��CH3��COOH��������B��C�Ľṹ��ʽ���Ƴ�AΪ ��
��
(1)�������Ϸ�����֪A�Ľṹ��ʽΪ ��B�Ľṹ��ʽΪ(CH3)2CHCH2OH ��
��B�Ľṹ��ʽΪ(CH3)2CHCH2OH ��
�𰸣� (CH3)2CHCH2OH
(CH3)2CHCH2OH
(2)CΪ  ��(I)�������࣬�ܷ���������Ӧ��ȷ��Ϊ����ij����������ֻ��һ��ȡ����������������C��һ��ͬ���칹��Ľṹ��ʽ
��(I)�������࣬�ܷ���������Ӧ��ȷ��Ϊ����ij����������ֻ��һ��ȡ����������������C��һ��ͬ���칹��Ľṹ��ʽ![]() ��
��
�𰸣�![]()
(3)��Ӧ���� (CH3)2CHCHO����Ϊ (CH3)2CHCOOH�Ĺ��̣������Ƿ���ȫ��ֻ������Ƿ���ȫ�����ɣ�ע����Ի����������ɵ���Ҫ�к���ȫ������Ϊȡ��������������������������Һ�к��ᣬ�ټ�������������ͭ����У�������ש��ɫ������˵����Ӧδ��ȫ��
�𰸣�ȡ��������������������������Һ�к��ᣬ�ټ�������������ͭ����У�������ש��ɫ������˵����Ӧδ��ȫ
(4)��Ӧ����(CH3)2CHCH2OH����Ϊ(CH3)2CHCHO����Ӧ������������Ӧ��
�𰸣�������Ӧ
(5)д����Ӧ����ǿ���Һ������ ����ȥ��Ӧ����ѧ����ʽ
����ȥ��Ӧ����ѧ����ʽ  +2NaOH
+2NaOH +NaBr+2H2O ��
+NaBr+2H2O ��
�𰸣�  +2NaOH
+2NaOH +NaBr+2H2O
+NaBr+2H2O
(6) E���Ժϳɸ߾�����ݽṹ��ʽCH2=C��CH3��COOH��֪�й�����̼̼˫������˷����Ӿ۷�Ӧ���߾���Ľṹ��ʽ ��
��
�𰸣�

 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�