��Ŀ����
��2014�����ʡ��ԭ��У������ѧ�ڵ�һ��������ѧ�Ծ���
����������һ�ֹ�ҵ�Σ���������������Ӧ�ù㷺��������ͼ��ʾ�������г�װ����ʡ�ԣ���ҩƷ��̽���������������ᷴӦ�������������ijɷ֡���֪��
��NO��NO2��2OH-��2NO2-��2H2O
������Һ�����¶ȣ�NO2 21�桢NO ��152��
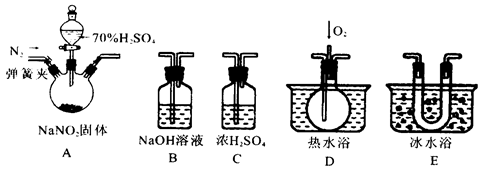
��1��Ϊ�˼���װ��A�����ɵ�����������������˳��Ϊ�������������ӣ���A��C��_______��_______��_______����װ���������������еIJ�����________________��
��2���رյ��ɼУ���Һ©������������70���������, A�в�������ɫ����.
��ȷ��A�в������庬��NO�����ݵ�������_____________________________.
��װ��E��������_______________________________________________________
��3�������D��ͨ�����O2,��װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ________________.
���û��װ��C����ʵ�������ɵ�Ӱ����______________________________��
��4��ͨ������ʵ��̽�����̣��ɵó�װ��A�з�Ӧ�Ļ�ѧ����ʽ��_______________��
��5����ҵ�����е���������ŷŻ���ɻ�����Ⱦ���ɲ������·��������������
CH4��g����2NO2��g����N2��g����CO2��g����2H2O��g�� ��H����867kJ��mol-1
CH4��g����4NO��g����2N2��g����CO2��g����2H2O��g�� ��H����1160kJ��mol-1
��CH4��NO2��ԭΪNO���Ȼ�ѧ����ʽΪ��___________________________.
����������һ�ֹ�ҵ�Σ���������������Ӧ�ù㷺��������ͼ��ʾ�������г�װ����ʡ�ԣ���ҩƷ��̽���������������ᷴӦ�������������ijɷ֡���֪��
��NO��NO2��2OH-��2NO2-��2H2O
������Һ�����¶ȣ�NO2 21�桢NO ��152��

��1��Ϊ�˼���װ��A�����ɵ�����������������˳��Ϊ�������������ӣ���A��C��_______��_______��_______����װ���������������еIJ�����________________��
��2���رյ��ɼУ���Һ©������������70���������, A�в�������ɫ����.
��ȷ��A�в������庬��NO�����ݵ�������_____________________________.
��װ��E��������_______________________________________________________
��3�������D��ͨ�����O2,��װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ________________.
���û��װ��C����ʵ�������ɵ�Ӱ����______________________________��
��4��ͨ������ʵ��̽�����̣��ɵó�װ��A�з�Ӧ�Ļ�ѧ����ʽ��_______________��
��5����ҵ�����е���������ŷŻ���ɻ�����Ⱦ���ɲ������·��������������
CH4��g����2NO2��g����N2��g����CO2��g����2H2O��g�� ��H����867kJ��mol-1
CH4��g����4NO��g����2N2��g����CO2��g����2H2O��g�� ��H����1160kJ��mol-1
��CH4��NO2��ԭΪNO���Ȼ�ѧ����ʽΪ��___________________________.
(1)E D B(2��)������װ�õ������ԣ�1�֣� (2)��D�г��ֺ���ɫ����(2��) ��������ʹNO2��ȫҺ��2�֣�(3)4NO2+O2+4NaOH=4NaNO3+2H2O(2��) ˮ����NO2��Ӧ����NO��Ӱ��NO��ȷ��(2 ��)
(4)2NaNO2+H2SO4=Na2SO4+NO2��+NO��+H2O(2��)
(5) CH4��g����4NO2��g����4NO(g)��CO2��g����2H2O��g�� ��H����574kJ��mol-1(2��)
������ʵ���⡣ʵ������ͬѧ�ǵ��ѵ㡣Ҳ�����ѵ÷ֵ����͡�����ʵ���⣬�����迴���ʵ��Ŀ����ʲô���ڽ�Ͽ���ʵ�����̣�ʵ����������ʼ����֪ʶ�ᴩ�����С�������һ��ʵ��̽���⣬̽��NaNO2��H2SO4�ķ�Ӧ��������װ���У�A��Ȼ�Ƿ���װ�á�B��NaOH��Һ�������dz���װ�ã�C��Ũ���ᣬ��Ȼ�Ǹ���װ�á�D����ˮԡ����ͨ����O2����ҪͨO2�������NO���塣E����ˮԡװ�á���Ȼ����ȴ���ʡ�����������2����Ϣ����һ����Ϣ��NO��NO2�Ļ��������NaOH�������з�Ӧ���ڶ�����Ϣ��NO��NO2����������������ʡ���Һ�����¶���������ȻNO2��NO����Һ����A������ƿ�����ù�Һ�����ȷ�Ӧ������һ�����ɼ���������ͨ��������ȻҪ������Ŀ��Ӧ���ų��������������������ԡ����ܻ��ʵ�����������š�ͨ��������Ϣ�Ϳ��Բ²���������������������NO��NO2��
��1��Ϊ�˼���װ��A�����ɵ�����������������˳��Ϊ�������������ӣ���
���Ƿ���װ�ã����Ǹ����ȥˮ��NO��NO2�ķ��룬�ȷ���NO2��Ϊ����Һ�������Խ�E��NO2������ټ���NO����ͨ��������D���۲쵽�������ɫ��ɺ���ɫ������˵����NO��B��β�����������շ�Ӧ��ʣ���NO��NO2�Ļ�����塣��������ڼ����Ⱥ����⣬����NO��NO2�ļ��飬����Һ���¶��жϵõ�NO2����Һ�������Թ۲쵽E���к���ɫҺ�����������NO�����������NO2������NO2�ļ����������š������ȼ���NO2������һ������dz�ˮ���������ȥˮ�Ļ���NO2����H2O��Ӧ����NO�������ֻ��NO�ļ���������ţ�ͬʱҲӰ��NO2�ļ��顣����һ���������A��ҩƷ��Ӧ֮ǰ��ͨ������O2�ų���������Ҳ��Ӱ��NO��NO2�ļ��顣װ�����Ӻ�֮���Ǽ��װ�������ԡ�
��2���رյ��ɼУ���Һ©������������70���������, A�в�������ɫ���塣˵��������NO2���塣
��ȷ��A�в������庬��NO������Dװ����ͨO2������ġ��۲������Ƿ�����ɫ��
��װ��E����������������ʹNO2��ȫҺ����
��3�������D��ͨ�����O2,������NOʣ�࣬���й�����O2����NO2����������ԭ��Ӧ��O2���ͣ�NO2���ߣ����Կ��ƶϳ���Ӧ����ʽΪ��4NO2+O2+4NaOH=4NaNO3+2H2O���粻���ˮ����NO2��Ӧ����NO��Ӱ��NO��ȷ����
��4��ͨ������ʵ��̽�����̣��ɵó�װ��A�з�ӦӦ�����������塣+3�۵�Ԫ�ط����绯��Ӧ����ѧ����ʽ��2NaNO2+H2SO4=Na2SO4+NO2��+NO��+H2O��
��5����ҵ�����е���������ŷŻ���ɻ�����Ⱦ���ɲ������·��������������
CH4��g����2NO2��g����N2��g����CO2��g����2H2O��g�� ��H����867kJ��mol-1
CH4��g����4NO��g����2N2��g����CO2��g����2H2O��g�� ��H����1160kJ��mol-1
���ݸ�˹������ʽ��2-��ʽ�ɵó���CH4��g����4NO2��g����4NO(g)��CO2��g����2H2O��g�� ��H����574kJ��mol-1(2��)

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 2NO(g) ��H=+180.5kJ��mol-1
2NO(g) ��H=+180.5kJ��mol-1 
 ��ͼ�е�
��ͼ�е� ��ʾ����1mol��������ݣ���������ͼ�ش��������⣺
��ʾ����1mol��������ݣ���������ͼ�ش��������⣺
 ���Ȼ�ѧ����ʽΪ ��
���Ȼ�ѧ����ʽΪ �� = KJ��mol-1
= KJ��mol-1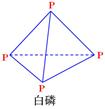 ��P4O10��
��P4O10��
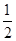 O2(g)=H2O(l) ��H����286 kJ/mol���ж�H2O������O��H���ļ���Ϊ( )
O2(g)=H2O(l) ��H����286 kJ/mol���ж�H2O������O��H���ļ���Ϊ( )