题目内容
(14分)化学在能源开发与利用中起着十分重要的作用。
(1)蕴藏在海底的可燃冰是高压下形成的外观像冰的甲烷水合物固体.被称之为“未来能源”。在25℃、101 kPa下,1g甲烷完全燃烧生成和液态水时放热55.6 kJ。甲烷燃烧的热化学方程式为 ______:相同条件下,356 g可燃冰(分子式为CH4·9H2O,Mr=178)释放的甲烷气体完全燃烧生成CO2和液态水,放出的热量为_______kJ。
(2)二甲醚(CH3OCH3)是无色气体,可作为一种新型能源,具有清洁、高效的优良性能。由合成气(组成为H2、CO和少量的CO2)直接制各二甲醚,其中的主要过程包括以下四个反应:
甲醇合成反应:
(Ⅰ)CO(g)+2H2(g)═CH3OH(g) △H1=-90.1kJ?mol-1
(Ⅱ)CO2(g)+3H2(g)═CH3OH(g)+H2O(g) △H2=-49.0kJ?mol-1
水煤气变换反应:(Ⅲ)CO(g)+H2O(g)═CO2(g)+H2 (g) △H3=-41.1kJ?mol-1
二甲醚合成反应:(Ⅳ)2CH3OH(g)═CH3OCH3(g)+H2O(g)△H4=-24.5kJ?mol-1
①分析二甲醚合成反应(iv)对于CO转化率的影响___________________________________。
②由H2和CO直接制备二甲醚(另一产物为水蒸气)的热化学方程式为:__________________。根据化学反应原理,分析增加压强对直接制备二甲醚反应的影响_________________________________。
(3)二甲醚直接燃料电池具有启动快、效率高等优点。若电解质为碱性,二甲醚直接燃料电池的负极反应为______________________,一个二甲醚分子经过电化学氧化,可以产生________电子的电量。
(1)蕴藏在海底的可燃冰是高压下形成的外观像冰的甲烷水合物固体.被称之为“未来能源”。在25℃、101 kPa下,1g甲烷完全燃烧生成和液态水时放热55.6 kJ。甲烷燃烧的热化学方程式为 ______:相同条件下,356 g可燃冰(分子式为CH4·9H2O,Mr=178)释放的甲烷气体完全燃烧生成CO2和液态水,放出的热量为_______kJ。
(2)二甲醚(CH3OCH3)是无色气体,可作为一种新型能源,具有清洁、高效的优良性能。由合成气(组成为H2、CO和少量的CO2)直接制各二甲醚,其中的主要过程包括以下四个反应:
甲醇合成反应:
(Ⅰ)CO(g)+2H2(g)═CH3OH(g) △H1=-90.1kJ?mol-1
(Ⅱ)CO2(g)+3H2(g)═CH3OH(g)+H2O(g) △H2=-49.0kJ?mol-1
水煤气变换反应:(Ⅲ)CO(g)+H2O(g)═CO2(g)+H2 (g) △H3=-41.1kJ?mol-1
二甲醚合成反应:(Ⅳ)2CH3OH(g)═CH3OCH3(g)+H2O(g)△H4=-24.5kJ?mol-1
①分析二甲醚合成反应(iv)对于CO转化率的影响___________________________________。
②由H2和CO直接制备二甲醚(另一产物为水蒸气)的热化学方程式为:__________________。根据化学反应原理,分析增加压强对直接制备二甲醚反应的影响_________________________________。
(3)二甲醚直接燃料电池具有启动快、效率高等优点。若电解质为碱性,二甲醚直接燃料电池的负极反应为______________________,一个二甲醚分子经过电化学氧化,可以产生________电子的电量。
(1)2O2(g)+CH4(g)═CO2(g)+H2O(l) △H=-889.6kJ/mol 1779.2
(2)①消耗甲醇,促进甲醇合成反应(Ⅰ)平衡右移,CO转化率增大;生成的H2O,通过水煤气变换反应(Ⅲ)消耗部分CO
②2CO(g)+4H2(g)=CH3OCH3(g)+H2O(g) △H=-204.7kJ?mol-1
该反应分子数减少,压强升高使平衡右移,CO和H2转化率增大,CH3OCH3产率增加.压强升高使CO和H2浓度增加,反应速率增大
(3)CH3OCH3+16OH--12e-=2CO32-+11H2O;12
(2)①消耗甲醇,促进甲醇合成反应(Ⅰ)平衡右移,CO转化率增大;生成的H2O,通过水煤气变换反应(Ⅲ)消耗部分CO
②2CO(g)+4H2(g)=CH3OCH3(g)+H2O(g) △H=-204.7kJ?mol-1
该反应分子数减少,压强升高使平衡右移,CO和H2转化率增大,CH3OCH3产率增加.压强升高使CO和H2浓度增加,反应速率增大
(3)CH3OCH3+16OH--12e-=2CO32-+11H2O;12
试题分析:(1)1g甲烷完全燃烧生成和液态水时放热55.6 kJ,则16g甲烷即1mol甲烷完全燃烧生成和液态水时放热55.6 kJ×16=889.6kJ,因此甲烷燃烧的热化学方程式为2O2(g)+CH4(g)═CO2(g)+H2O(l) △H=-889.6kJ/mol。356 g可燃冰(分子式为CH4·9H2O,Mr=178)中甲烷的质量是356g×
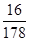 =32g,即含有2mol甲烷,所以释放的甲烷气体完全燃烧生成CO2和液态水,放出的热量为889.6kJ/mol×2mol=1779.2kJ。
=32g,即含有2mol甲烷,所以释放的甲烷气体完全燃烧生成CO2和液态水,放出的热量为889.6kJ/mol×2mol=1779.2kJ。(2)①二甲醚合成反应(Ⅳ)对于CO转化率的影响,消耗甲醇,促进甲醇合成反应(Ⅰ)CO(g)+2H2(g)═CH3OH(g)平衡右移,CO转化率增大;生成的H2O,通过水煤气变换反应(Ⅲ)CO(g)+H2O(g)═CO2(g)+H2 (g)消耗部分CO,故答案为:消耗甲醇,促进甲醇合成反应(Ⅰ)平衡右移,CO转化率增大;生成的H2O,通过水煤气变换反应(Ⅲ)消耗部分CO;
②Ⅰ、CO(g)+2H2(g)═CH3OH(g)△H1=-90.1kJ?mol-1、Ⅳ、2CH3OH(g)═CH3OCH3(g)+H2O(g)△H4=-24.5kJ?mol-1,依据盖斯定律Ⅰ×2+Ⅳ得到:2CO(g)+4H2(g)=CH3OCH3+H2O(g) △H=-204.7kJ?mol-1。该反应是气体体积减小的反应,增加压强平衡正向进行,反应速率增大,CO和H2转化率增大,CH3OCH3产率增加;故答案为:2CO(g)+4H2(g)=CH3OCH3(g)+H2O(g)△H=-204.7kJ?mol-1;该反应分子数减少,压强升高使平衡右移,CO和H2转化率增大,CH3OCH3产率增加.压强升高使CO和H2浓度增加,反应速率增大;
(3)原电池中负极失去电子,则二甲醚在负极发生氧化反应。若电解质为碱性,二甲醚直接燃料电池的负极反应为二甲醚失电子生成碳酸根,结合原子守恒和电荷守恒写出电极反应为:CH3OCH3+16OH--12e-=2CO32-+11H2O; 根据负极电极反应式可知一个二甲醚分子经过电化学氧化,可以产生12个电子的电量。

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目
 O2(g) ΔH=-266 kJ·mol-1
O2(g) ΔH=-266 kJ·mol-1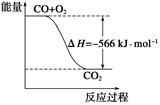
 2NH3为放热反应,由此推断出 ( )
2NH3为放热反应,由此推断出 ( ) CH3OH(g)
CH3OH(g) 


 MnO4-+
MnO4-+