��Ŀ����
����Ŀ��(1)���г������ʣ�
A.̼������ B.��˹ƥ�� C.������ D.�������� E.���ʻ�
������ĸ��գ�
�پ��н�����ʹ��Ч����________���ڿ�ֱ�ӽ�������ѪҺ������������________���ۿ�����ʳƷ����������________���ܳ�����ʳƷ��ɫ������________���ݳ���������θ�����Ŀ��������________��
(2)������������������
�ٿ�����������У�����Ҫ����ָ����_________��
A.�����������ĺ��� B.NO2Ũ�� C.SO2Ũ�� D.CO2Ũ��
���ڴ��������ķ�ˮʱ�����ȼ�����������������____________����ͨ��������������ȣ���������______________��
�۶�������Ҫ���ദ������ͼ��ʾ�����������־�ĺ�����___________��

(3)��ѧ��������й�����
��ʯīϩ(��ͼ)������̫���ܵ�صĵ缫,������Ҫ������ʯīϩ��________�ԡ�

�ڸ����������лᷢ���绯ѧ��ʴ�����һ������������ʩ____________��
���𰸡�B C D E A D ������ ɱ������ ѭ����������� ���� ���ɲ���ַ���ʴ
��������
(1)���ݸ����ʵ����ʷ�����
(2)�ٶ�����̼�͵����ǿ����ijɷ֣���������Ⱦ�
����������ˮʱ�������������ˮ�����������������壬���������Կ��Ծ�ˮ��������������Ⱦ���ǿ�����ԣ�������ɱ����
�۸���ͼ����ʾ��־�ĺ����жϣ�
(3)��ʯīϩ����ʮ�����õ�ǿ�ȡ����͡����硢���ȡ���ѧ���Եȣ����缫��������ʯīϩ�ĵ����ԣ�
���ڸֲ������Ӹ��������ɲ���ַ���ʴ��
(1)A.̼��������ˮ����ˮ�⣬ʹ��Һ�е����������ӵ�Ũ�ȴ��������ӵ�Ũ�ȣ�����̼�����Ƶ�ˮ��Һ�ʼ��ԣ����ܺ��ᷴӦ��
B.��˹ƥ���Ǹ�ðҩ�����н�����ʹ���ã�
C.�������������ܱ������ֽ⣬�������ɶ�����̼��ˮ��ͬʱ�ͷ������������ܸ����岹��������
D.���������dz��õķ�������
E.���ʻơ���֬�졢���ܲ��ص��dz��õ���ɫ����
��پ��н�����ʹ��Ч����B���ڿ�ֱ�ӽ�������ѪҺ������������C���ۿ�����ʳƷ����������D���ܳ�����ʳƷ��ɫ������E���ݳ���������θ�����Ŀ��������A��
(2)�ٿ�����Ⱦ��;����Ҫ���������к�����ͷ۳����к�������Ҫ��һ����̼���������������������壻�۳���ҪָһЩ����С������������̼�͵����ǿ����ijɷ֣���������Ⱦ������ڿ�����������У�����Ҫ����ָ����D��
����������ˮʱ�������������ˮ�����������������壬���������Կ��Ծ�ˮ�����������ǻ�������������������Ⱦ���ǿ�����ԣ�����ɱ���������ã�
��ͼ����ʾ��־�ǻ��ձ�־���ʺ�������ѭ����������գ�
(3)��ʯīϩ��һ����̼ԭ����sp2�ӻ���ʽ�γɵķ���״ƽ�污Ĥ����һ��ֻ��һ��ԭ�Ӳ��ȵ���ά���ϣ������ֽ�����ԭ�Ӳ�ʯī��ʵ����ʯīϩ�����ʹ�������Ȼ�磬ֻ�������������ṹ��ʯīϩһ������������ʯī��Ǧ����ֽ�����Ữ�������µĺۼ��Ϳ����Ǽ�����������һ��ʯīϩ��ʯīϩ����ʮ�����õ�ǿ�ȡ����͡����硢���ȡ���ѧ���Եȣ����缫��������ʯīϩ�ĵ����ԣ�
���ڸֲ������Ӹ���ʹ�ֲı����γ������Ҽ�̵�����Ĥ����ֹ�ڲ������������Ӵ�����������߸ֵĿ������Ժ���ʴ�ԣ���������ʹ��������ᡢ�ʴ�����ڸֲ������Ӹ�������Ԫ�ص�Ŀ�������ɲ���ַ���ʴ��

 ������������ϵ�д�
������������ϵ�д�����Ŀ��������ͭ�Ǻϳ��������������в���������������ͭ����Ҫǰ����֮һ������������һ��ʵ���Һϳ�·�ߣ�
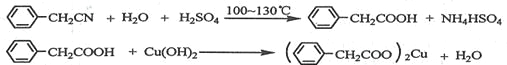
�Ʊ��������װ��ʾ��ͼ����(���Ⱥͼг�װ�õ���)��
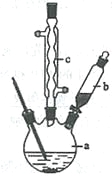
��֪����������۵�Ϊ76.5 �棬������ˮ�������Ҵ���
�ش��������⣺
��1����250 mL����ƿa�м���70 mL70%���ᡣ���ƴ�����ʱ����������ˮ��Ũ������Ⱥ�˳����
__________________________��
��2����a�е���Һ������100 �棬�����μ�40 g�����浽������Һ�У�Ȼ��������130 �������Ӧ����װ���У�����b��������_____________________������c��������______________����������___________________________________________��
��Ӧ�������������ˮ���ٷ�����������Ʒ��������ˮ��Ŀ����____________�����������п����ڷ��뱽�����Ʒ����________________(����)��
A����Һ©�� | B��©�� | C���ձ� | D��ֱ��������E�������� |
��3���ᴿ�ֱ�����ķ�����_____________�����յõ�44 g��Ʒ��������IJ�����________��
��4����CuCl2 2H2O��NaOH��Һ�Ʊ�����Cu(OH)2�����������������ˮϴ�ӳ������жϳ���ϴ�ɾ���ʵ�������������____________________________________________��
��5������������˵��Ҵ���ˮ�Ļ���ܼ��У�����ܽ����Cu(OH)2����30min�����ˣ���Һ����һ��ʱ�䣬����������ͭ���壬����ܼ����Ҵ���������___________________��
����Ŀ��һ���¶��£������������Ϊ0.5 L�ĺ����ܱ������з�����Ӧ��CO(g)+Cl2(g)![]() COCl2(g)�������������з�Ӧ��5 minʱ�ﵽƽ��״̬��
COCl2(g)�������������з�Ӧ��5 minʱ�ﵽƽ��״̬��
������� | �¶�/�� | ��ʼ���ʵ���/mol | ƽ�����ʵ���/mol | ||
CO | Cl2 | COCl2 | COCl2 | ||
�� | 500 | 1.0 | 1.0 | 0 | 0.8 |
�� | 500 | 1.0 | a | 0 | 0.5 |
�� | 600 | 0.5 | 0.5 | 0.5 | 0.7 |
����˵������ȷ����
A. ��������ǰ5 min��ƽ����Ӧ����v(CO)=0.16 mol��L-1��min-1
B. �÷�Ӧ����ӦΪ���ȷ�Ӧ
C. ����������ʼʱCl2�����ʵ���Ϊ0.55 mol
D. ����ʼʱ�����������CO0.8mol��Cl20.8mol���ﵽƽ��ʱCOת���ʴ���80%