��Ŀ����
���к͵ζ����ⶨ�ռ�Ĵ��ȣ����ռ��в��������ᷴӦ�����ʣ��Ը���ʵ��ش�
��ȷ��ȡ�ռ���Ʒ4.100g������Ʒ���250mL�Ĵ���Һ����Ҫ��������С�ձ�����Ͳ���������� __________��__________��___________ ��(������)
��ȡ10.00mL����Һ����__________��ȡע����ƿ�С�(������)
����0.2010mol/L��������Һ�ζ������ռ���Һ���ζ�ʱ����__________������__________������ע��__________��ֱ���ζ��յ㡣
��4���������вⶨ���ݣ������õ��������ݣ���������ռ���Һ��Ũ�ȣ�__________��
�ɸ��������ⶨ���ݣ������õ��������ݣ������ռ�Ĵ���__________��
��ȷ��ȡ�ռ���Ʒ4.100g������Ʒ���250mL�Ĵ���Һ����Ҫ��������С�ձ�����Ͳ���������� __________��__________��___________ ��(������)
��ȡ10.00mL����Һ����__________��ȡע����ƿ�С�(������)
����0.2010mol/L��������Һ�ζ������ռ���Һ���ζ�ʱ����__________������__________������ע��__________��ֱ���ζ��յ㡣
��4���������вⶨ���ݣ������õ��������ݣ���������ռ���Һ��Ũ�ȣ�__________��
| �ζ����� | ����Һ���/mL | ���������/mL | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 10.00 | 0.50 | 20.52 |
| �ڶ��� | 10.00 | 4.00 | 23.08 |
| ������ | 10.00 | 4.20 | 26.70 |
��14�֣���1��250mL����ƿ����ͷ�ιܡ���ƽ�ȣ�3�֣�
��2�����ü�ʽ�ζ�������ȡ��Ҳ��ʹ����Һ������ȡ��2�֣�
��3���ζ������У��ζ�ʱ���ֿ��ƻ��������ֲ�ͣ������ƿ������ע����ƿ����Һ��ɫ�ı仯��ֱ���ζ��յ㡣��3�֣�
��4��V(NaOH)=10.00mL V(HCl)=[(20.52mL-0.50mL)+(23.08mL-4.00)]/2=20.00mL
���ݣ�c(NaOH)V(NaOH)=c(HCl)V(HCl) ��c(NaOH)=0.4020mol/L��3�֣�
��5����Ʒ��m(NaOH)=0.4020mol/L��0.250L��40g/mol=4.02g
���ռ�Ĵ���Ϊ��4.02g/4.1g��100%=98.05% ��3�֣�
��2�����ü�ʽ�ζ�������ȡ��Ҳ��ʹ����Һ������ȡ��2�֣�
��3���ζ������У��ζ�ʱ���ֿ��ƻ��������ֲ�ͣ������ƿ������ע����ƿ����Һ��ɫ�ı仯��ֱ���ζ��յ㡣��3�֣�
��4��V(NaOH)=10.00mL V(HCl)=[(20.52mL-0.50mL)+(23.08mL-4.00)]/2=20.00mL
���ݣ�c(NaOH)V(NaOH)=c(HCl)V(HCl) ��c(NaOH)=0.4020mol/L��3�֣�
��5����Ʒ��m(NaOH)=0.4020mol/L��0.250L��40g/mol=4.02g
���ռ�Ĵ���Ϊ��4.02g/4.1g��100%=98.05% ��3�֣�
�����������1�����250mL�Ĵ���Һ����Ҫ��������С�ձ�����Ͳ���������⣬����250mL����ƿ����ͷ�ιܡ���ƽ�ȡ�
��2������������Һ�Լ��ԣ�����ȡ10.00mL����Һ��Ӧ���ü�ʽ�ζ�������ȡ��Ҳ��ʹ����Һ������ȡע����ƿ�С�
��3����0.2010mol/L��������Һ�ζ������ռ���Һ���ζ�ʱ���ֿ��ƻ��������ֲ�ͣ������ƿ������ע����ƿ����Һ��ɫ�ı仯��ֱ���ζ��յ㡣
��4�����ݱ������ݿ�֪������ʵ������������������20.02ml��19.08ml��22.5ml����Ȼ������ʵ�������̫����ȥ�����������������ƽ��ֵ�ǣ�20.02ml��19.08����2��20.00ml��������ռ���Һ��Ũ����
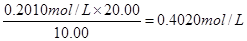
��5����Ʒ��m(NaOH)=0.4020mol/L��0.250L��40g/mol=4.02g
���ռ�Ĵ���Ϊ��4.02g/4.1g��100%=98.05%
�������������е��Ѷȵ����⣬���������ǿ���������У������߿���������ע�ض�ѧ��������֪ʶ������ѵ����ͬʱ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ�������������������Ӧ������������ѧ����ѧ��������

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
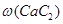 ��_______________��
��_______________��
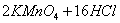 (Ũ)="==="
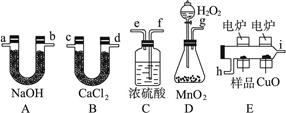
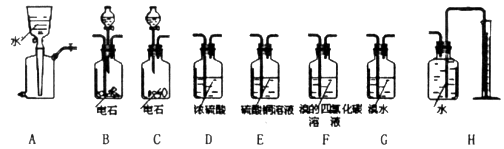
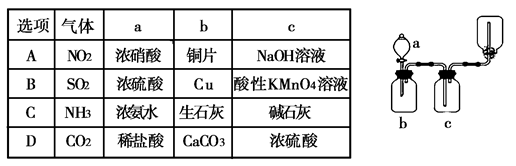
(Ũ)="===" 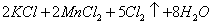 ����ʵ���ҿ��ö������̹���������ع����Ũ���ᷴӦ��ȡ�������ɹ�ѡ�õķ���װ������ͼ��
����ʵ���ҿ��ö������̹���������ع����Ũ���ᷴӦ��ȡ�������ɹ�ѡ�õķ���װ������ͼ��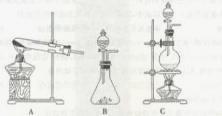
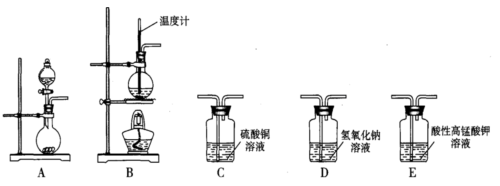
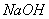 ��Һ����������ֹ��Ⱦ,д���÷�Ӧ�����ӷ���ʽ ��
��Һ����������ֹ��Ⱦ,д���÷�Ӧ�����ӷ���ʽ ��