��Ŀ����
����Ŀ�������仯������������˽ũҵ��������Ҫ��������NAΪ�����ӵ�������ֵ�������й�˵��������ǣ� ��
A.��״���£�5.6LNO��5.6LO2��ֻ�Ϻ�ķ�����Ϊ0.5NA
B.��״���£�22.4L15NH3���е�������Ϊ10NA
C.13.8gNO2������ˮ��Ӧ��ת�Ƶĵ�����Ϊ0.2NA
D.�����£�1L0.1mol��L1NH4NO3��Һ�к��еĵ�ԭ����Ϊ0.2NA
���𰸡�A
��������
A. ��״���£�5.6LNO�����ʵ���Ϊ0.25mol��5.6LO2�����ʵ���Ϊ0.25mol�����߳�ֻ�Ϻ���2NO ��O2 ��2NO2�����ݷ���ʽ��ϵ������O2�����ʵ���Ϊ0.125mol��NO��Ӧ�꣬����0.25mol NO2���ܵ����ʵ���Ϊ0.25 mol��0.125mol��0.375mol�����ڶ������������Է��������淴Ӧ����������������������ʵ���С��0.375mol��������С��0.375NA����A����
B. 1��15NH3����10�����ӣ���״���£�22.4L15NH3�����ʵ���Ϊ1mol�����22.4L15NH3 ���е�������Ϊ10NA����B��ȷ��
C. NO2������ˮ��Ӧ3 NO2��H2O ��2HNO3��NO��3mol NO2ת��2mol���ӣ�13.8gNO2�����ʵ���![]() �����13.8gNO2������ˮ��Ӧת�Ƶĵ�����Ϊ0.2NA����C��ȷ��
�����13.8gNO2������ˮ��Ӧת�Ƶĵ�����Ϊ0.2NA����C��ȷ��
D. �����£�1L0.1mol��L1NH4NO3��Һ���ʵ���Ϊ![]() ����˺��еĵ�ԭ����Ϊ0.2 NA����D��ȷ��
����˺��еĵ�ԭ����Ϊ0.2 NA����D��ȷ��
������������ΪA��

 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�����Ŀ�������ѧ���������ɫ���ɡ����롣�ѿ����е�CO2����ת������ʹ֮��H2��Ӧ���ɿ�������Դ�״���
��1����ҵ�����п�����H2��ԭCO2�Ʊ������Դ�״���
�����й������ܵ�˵����ȷ����____��
A�������������Դ B�������Ƕ�����Դ
C�������Dz���������Դ D����̬����Դ�����ױ��������
����֪CO��g����H2��g����ȼ���ȣ���H���ֱ�Ϊ-283.0kJmol-1��-285.8kJmol-1��CO��H2�ϳɼ״��������仯��ͼ����ʾ��
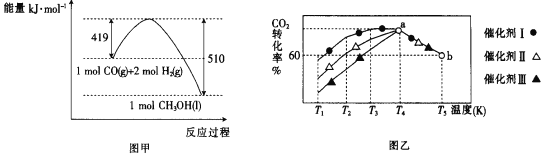
����CO2��g����H2��g���Ʊ�Һ̬�״����Ȼ�ѧ����ʽΪ____��
�۽�һ������CO2��H2���뵽ij�����ܱ������У�����ڲ�ͬ���������£���ͬʱ����CO2��ת�������¶ȵı仯��ͼ����ʾ����Ч����õ��Ǵ���____��ѡ�������
��2������CO��ˮ����������H2����Ӧ�Ļ�ѧ����ʽ��CO��g��+H2O��g��CO2��g��+H2��g��������ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������н������·�Ӧ���õ��������������ʾ��
�¶�/�� | ��ʼ�� | �ﵽƽ�� | |||
CO/mol | H2O/mol | H2/mol | COת���� | ʱ��/min | |
650 | 4 | 2 | 1.6 | 10 | |
900 | 3 | 2 |
| 5 | |
900��ʱ���ﵽƽ��ʱ�ķ�Ӧ����v��H2O��=____��������2λС������
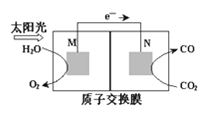
��3����ѧ�һ��о�������ת����������ķ�����������ͼ��ʾװ�ÿ��Խ�CO2ת��Ϊ����ȼ��CO����װ�ù���ʱ��N�缫�ĵ缫��ӦʽΪ____��