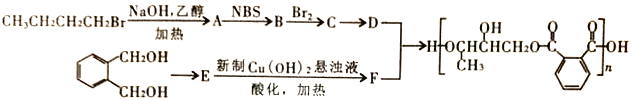
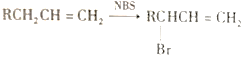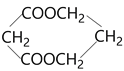��Ŀ����
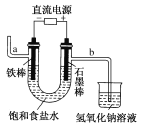
����Ŀ���������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�á�
��1��д���������������ƣ�a��__________�� b��___________��
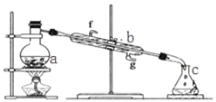
��2��ʵ������У���Ҫͨ��ˮ��ͼ�еĽ�ˮ������_______������ͼ����ĸ����
��3��������װ�÷������ᣨ�е�118�棩�������������е�77�棩�Ļ�����ȱ�ٵ�������_______��
II������NaOH��������0.1 mol/L NaOH��Һ480mL���ݴ˻ش��������⣺
��4����������������Һ��Ҫ�õ��������������ձ�������������ͷ�ιܺ�______��
��5��ʵ��ʱ��Ҫ������������_______g��
��6������0.1 mol/L NaOH��Һ��ʵ���У�����������²������ᵼ��������Һ��Ũ��ƫ�����_______����д��ĸ����
A�������������ƹ���ʱ����ŷ��� B��δϴ���ܽ�NaOH���ձ�
C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ��
D������ƿδ���T����������Һ E������ʱ���ӿ̶���
F�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶���
���𰸡�������ƿ ������ g �¶ȼ� 500mL ����ƿ 2.0 g CE
��������
��1�����������Ĺ���������ƣ�
��2����ˮ�½��ϳ�������������ͣ��ʱ�䳤����ȴЧ���ã�
��3���������ᣨ�е�118�棩�������������е�77�棩�Ļ�����ȡ������Ҫ�¶ȼƲⶨ�¶ȣ�
II����4������0.1 mol/L NaOH��Һ480mL��ѡ��500mL����ƿ�����ձ����ܽ⡢��ȴ��ת�Ƶ�����ƿ�ж��ݣ�
��5�����m=cVM���㣻
��6�����c=n/V��֪��������������nƫ���VƫС���ᵼ��������Һ��Ũ��ƫ���Դ������
��1������a��b�����Ʒֱ�Ϊ������ƿ�������ܣ�
��2��ʵ������У���Ҫͨ��ˮ��ͼ�еĽ�ˮ������g����f����
��3�����ᣨ�е�118�棩�������������е�77�棩���ܣ����ߵķе����ϴ�����ߵĻ�����ȡ������Ҫ�¶ȼƲⶨ�¶ȣ�ͼ��ȱ�ٵ�����Ϊ�¶ȼƣ�
II����4������0.1molL-1NaOH��Һ480mL��ѡ��500mL����ƿ�����ձ����ܽ⡢��ȴ��ת�Ƶ�����ƿ�ж��ݣ�����Ҫ�õ��IJ�����������Ͳ���ձ�������������ͷ�ιܡ�500mL����ƿ���ʻ�ȱ��500mL����ƿ��
��5��ʵ��ʱ��Ҫ�����������Ƶ�����Ϊ0.5L��0.1mol/L��40g/mol=2.0g��
��6��A�������������ƹ���ʱ����ŷ��ˣ������ʹ�����룬������������䣬Ũ�Ȳ��䣬���ʹ�����룬���������٣�Ũ��ƫС��
B��δϴ���ܽ�NaOH���ձ���nƫС��Ũ��ƫС��
C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�У���ȴ��VƫС����Ũ��ƫ��
D������ƿδ���T����������Һ����ʵ����Ӱ�죻
E������ʱ���ӿ̶��ߣ�VƫС����Ũ��ƫ��
F�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�Vƫ����Ũ��ƫС��
�ʴ�ΪCE��