��Ŀ����
����Ŀ��ʵ����Ҫ����480mL0.1mol��L-1Na2CO3��Һ���ش��������⡣
��1��Ӧ��������ƽ��ȡNa2CO3��10H2O����___g��
��2������Na2CO3��Һʱ���õ���Ҫ�����������ձ�����������___��___��
��3����ʵ���������������Һ��Ũ��ƫ�͵���___
A����ˮʱ�����̶���
B�����ǽ�ϴ��Һ��������ƿ
C������ƿ�ڱڸ���ˮ���δ���и��ﴦ��
D�����ݺ�ҡ�ȣ�Һ����ڿ̶���
E������ʱ��������
���𰸡�14.3g 500ml����ƿ ��ͷ�ι� AB
��������
��1������n=cv��������Na2CO3�����ʵ���������Na2CO310H2O�����ʵ�������Na2CO3�����ʵ���������m=nM����Na2CO310H2O��������
��2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ��������������
��3������c=![]() ���㲻��������n��V��Ӱ�죬���nƫ���VƫС������������ҺŨ��ƫ�ߡ�
���㲻��������n��V��Ӱ�죬���nƫ���VƫС������������ҺŨ��ƫ�ߡ�
��1����������Һ�����Ϊ480mL��������ƿ�Ĺ��û��480mL��ֻ��ѡ��500mL��
Na2CO3�����ʵ���n=cV=0.5L��0.1molL-1=0.05mol��Na2CO310H2O�����ʵ�������Na2CO3�����ʵ���������Na2CO310H2O������0.05mol��286g/mol=14.3g��
��2������˳���ǣ��������������ܽ⡢��ȴ����Һ��ϴ����������ҡ����װƿ��ǩ��һ������ƽ�������õ�ҩ�ף����������ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
��3��A����ˮʱ�����̶��ߣ���Һ�����ƫ��Ũ��ƫ�ͣ�ѡ��A��ȷ��
B�����ǽ�ϴ��Һת������ƿ��ϴ��Һ�к������ʣ����ʵ�����ƫС��Ũ��ƫ�ͣ�ѡ��B��ȷ��
C������ƿϴ�Ӻ�ײ��ڱ���ˮ���δ�����ﴦ������Ӱ����Һ�������Ũ�Ȳ��䣬ѡ��C����
D�����ݡ�ҡ�ȡ����ú��ְ�Һ����ڿ̶��ߣ�����������ڿ̶������ϵ���Һ�ͻ����䣬��Һ��������䣬Ũ����Ӱ�죬ѡ��D����
E������ʱ�������⣬���ƹ�������ƫ��������ҺŨ��ƫ��ѡ��E��ȷ��
��ѡAB��

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�����Ŀ����֪X��Y��Z��Ϊ��ѧ��ѧ�������ʣ��Ҿ�����ͬһ��Ԫ�أ�����X�ǵ��ʣ�����֮���ת����ϵ��ͼ��ʾ����X��Y��Z�������ǣ� ��
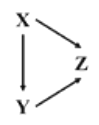
���� ѡ�� | X | Y | Z |
A | Na | Na2O | NaOH |
B | Fe | FeCl3 | FeCl2 |
C | Mg | Mg(OH)2 | MgO |
D | Si | SiO2 | Na2SiO3 |
A.AB.BC.CD.D
����Ŀ�����������Ҫ�ɷֿɱ�ʾΪFeO��Cr2O3��������MgO��Al2O3��Fe2O3�����ʣ��������Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7��������ͼ��

��֪����4FeO��Cr2O3+8Na2CO3+7O2![]() 8Na2CrO4+2Fe2O3+8CO2����
8Na2CrO4+2Fe2O3+8CO2����
��Na2CO3+Al2O3![]() 2NaAlO2+CO��
2NaAlO2+CO��
���������գ�
��1���������ڳ����¸÷�Ӧ���ʼ��������д�ʩ����ʹ��Ӧ�����������____��
A.�����¶� B.ͨ������Ŀ��� C.��ԭ�Ϸ��� D.���Ӵ��������
��2������X����Ҫ����___����д��ѧʽ����
��3�����������жಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ����___��____��ϴ�ӡ����
��4�������������ʵ��ܽ�����ݣ��������з�����Ӧ�Ļ�ѧ����ʽ�ǣ�Na2Cr2O7+2KCl=K2Cr2O7��+2NaCl���÷�Ӧ����Һ���ܷ�����������____������˵������
���� | �ܽ�ȣ�g/100gˮ�� | ||
0�� | 40�� | 80�� | |
KCl | 28 | 40.1 | 51.3 |
NaCl | 35.7 | 36.4 | 38 |
K2Cr2O7 | 4.7 | 26.3 | 73 |
Na2Cr2O7 | 163 | 215 | 376 |
��5������ƷY��Ҫ��������������������þ���������ܻ����P���������ʣ���ȷ����Y���������������ķ����dz�ȡ![]() ��Ʒ���������____����д�Լ������ܽ⡢���ˡ��ټ��루��ͨ�룩____����д�Լ������������ա���ȴ���������ø������
��Ʒ���������____����д�Լ������ܽ⡢���ˡ��ټ��루��ͨ�룩____����д�Լ������������ա���ȴ���������ø������![]() ��������Ʒ��������������������Ϊ___���ú�m��n�Ĵ���ʽ��ʾ����
��������Ʒ��������������������Ϊ___���ú�m��n�Ĵ���ʽ��ʾ����