��Ŀ����
����Ŀ������������йع涨���龰��ա�
��1������ͼ�dz�ȥ����Ĥ��þ��Ͷ��ϡ�����в���������������ʱ��ı仯��ϵ������ǰ�γ��ָ߷����ҪӰ��������________��
����ͼ�ǹ���������ø�Ĵ������·ֽ��������¶ȵı仯��ϵ�����ߺ�������½�����ҪӰ��������__��
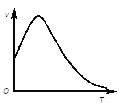
��2�����ù��ܺ�������ɽ�CO2��H2O(g)ת��ΪCH4��O2�����������ʱ���ڲ�ͬ������I��II��III�������£�CH4���������ʱ��ı仯��ͼ����0��15Сʱ�ڣ�CH4��ƽ����������I��II��III�Ӵ�С��˳��Ϊ_________������ţ���
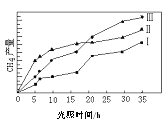
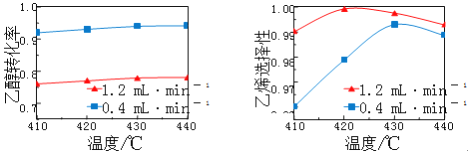
��3����TiO2��Cu2Al2O4Ϊ���������Խ�CO2��CH4ֱ��ת�������ᡣ�ڲ�ͬ�¶��´����Ĵ�Ч����������������ʵĹ�ϵ��ͼ��
��250��300��ʱ���¶����߶�������������ʽ��͵�ԭ����_____________��
�ڵ��¶ȵ�ȡֵ��Χ��________ʱ���¶���Ӱ��������������ʵ���Ҫ���ء�
���𰸡��¶� Ũ�� II��III��I �¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��� 300�桫400��
��������
���ͼ��۲����ʵı仯����Ӱ�����ʵ�������ط������
��1���ٸ���Ӱ�����ʵ����ط���������ԭ���Ƿ�Ӧ���ȣ��¶����ߵ������ʼӿ죬
�ʴ�Ϊ���¶ȣ�
�����ŷ�Ӧ���У���Ӧ��Ũ�Ƚ��ͣ����Է�Ӧ���ʼ�����
�ʴ�Ϊ��Ũ�ȣ�
��2�����ʿ����õ�λʱ���ڼ���IJ�������������ͼ����Կ�������0��15Сʱ�ڣ�CH4��ƽ����������I��II��III�Ӵ�С��˳��Ϊ��II��III��I��
�ʴ�Ϊ��II��III��I��
��3������֪�����Է�Ӧ��������Ҫ��ģ���250��300��ʱ���¶����߶�������������ʽ��͵�ԭ���ǣ��¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��ͣ�
��������������֪���¶ȹ���ʱ��������Ч�ʽ��ͣ���ͼ���֪300���Ժ����������¶������ֿ�ʼ���ߣ����Ե��¶ȵ�ȡֵ��Χ��300�桫400��ʱ���¶���Ӱ��������������ʵ���Ҫ���ء�
�ʴ�Ϊ���¶ȳ���250��ʱ�������Ĵ�Ч�ʽ����� 300�桫400�棻

����Ŀ��ijУ��ѧ��ȤС���ͬѧ�Ժ�������Na2SO4��NaOH��Ʒ��NaOH�ĺ������вⶨ���ش���������:
��1����ͬѧ���ó������ⶨ��Ʒ��NaOH�ĺ�������ͬѧѡ�õ�ҩƷ����Ʒ������ˮ��MgCl2��Һ����Ҫ�ⶨ��ʵ��������__________��
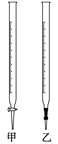
��2����ͬѧ�����к͵ζ����ⶨ��Ʒ��NaOH�ĺ�����
���÷�����ƽȷ��ȡ����Ʒ5.0000 g��ȫ������ˮ���Ƴ�1000.0 mL��Һ���ü�ʽ�ζ�����ȡ20.00 mL������Һ������ƿ�У��μӼ���ָʾ�������⡣�ζ�����ʹ��ǰ��ϴ���⣬��Ӧ____________________________________��
����Ũ��Ϊ0.100 0 mol��L��1���������Һ���еζ�����ʼ�ζ�ǰ��һ��������________��
�۵ζ���������pH�Ʋⶨ��ƿ����Һ��pH���ٽ��ζ��յ�ʱ�ⶨpHӦÿ��һ�β�һ�Ρ�
�ܵζ������У���ƿ����Һ��pH�仯���£�
V(HCl)/mL | 0.00 | 12.00 | 18.00 | 22.00 | 23.00 | 23.96 | 24.00 | 24.04 | 25.00 | 26.00 | 30.00 |
pH | 13.1 | 12.6 | 12.2 | 11.7 | 11.4 | 9.9 | 7.0 | 4.0 | 2.7 | 2.4 | 1.9 |
��������ͼ�л��Ƴ������к͵ζ������ߡ�______________

�������ʾ�Ǽ������ָʾ���ı�ɫ��Χ���������������к͵ζ����߷����������к͵ζ���Ӧѡ�õ�ָʾ����________��
ָʾ�� | ��ɫ��Χ(pH) | ��ɫ | |
�� | �� | ||
���� | 3.1��4.4 | �� | �� |
ʯ�� | 5.0��8.0 | �� | �� |
��̪ | 8.2��10.0 | �� | �� |
����Ʒ�У�NaOH�������ٷֺ���Ϊ____________��
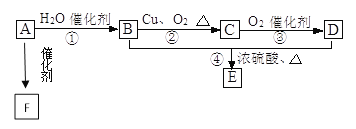
����Ŀ��������(AlN)��һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·���մɵ���������ҵ�����������뽹̿�ڵ������и����Ƶ�:Al2O3+3C+N2![]() 2AlN+3CO����ش�:
2AlN+3CO����ش�:
(1)������Ӧ���������뻹ԭ�������ʵ���֮��___________________��
(2)�Ƶõĵ�������Ʒ�г�����Al4C3��Al2O3��C�����ʣ���֪:
ϡ����(����) | ŨNaOH��Һ(����) | |
AlN | 2AlN+4H2SO4=Al2(SO4)3+(NH4)2SO4 | AlN+NaOH+H2O=NaAlO2+NH3 �� |
Al4C3 | Al4C3+6H2SO4=2Al2(SO4)3+3CH4 �� | Al4C3+4NaOH+4H2O=4NaAlO2+3CH4 �� |
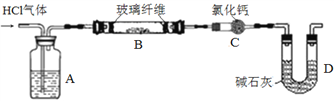
Ϊ�ⶨ��Ʒ��AlN��Al4C3�ĺ������������������ʵ��װ��(��ͨ������У����ʵ��ȣ����Կ�����ˮ������CO2��Ӱ�켰NH3��ǿ������Һ�е��ܽ�)��

װ��I��II�ڷ�Ӧǰ���������ֱ�Ϊm1 g��m2 g����m g��Ʒ��AlN����������Ϊ______(�ú�m��m1��m2�Ĵ���ʽ��ʾ)��
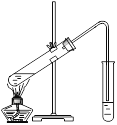
(3)ȡװ��I�з�Ӧ�����Һ���ȼ�������(NH4)2SO4��Һ��Ȼ�����ν�������Ũ������ȴ�ᾧ�����ˡ��������ƾ�ϴ�ӡ���ֽ���ɣ��õ����������[NH4Al(SO4)2��12H2O]��
������Ũ������ʱ����������̨(����Ȧ)���ƾ��ơ�����ǯ���������⣬����Ҫ_______(����������)������Ũ���IJ�����С����ȣ����Ͻ��裬_________��
���������ƾ�ϴ�ӵ�Ŀ��__________________________��
(4)��֪:25��ʱ��Kw=1.0��10-14��Kb(NH3��H2O)=1.8��10-5��Kb(Al(OH)3]=1.4��10-9��
�� NH4Al(SO4)2��Һ������Ũ���ɴ�С��˳��_________________��
��(NH4)2SO4��Һ�д���ˮ��ƽ�⣬��ˮ��ƽ�ⳣ��Ϊ_____________��