��Ŀ����
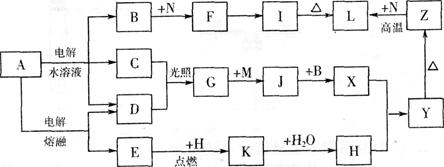
��14�֣�A��J����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ���¿�ͼ��ʾ�����ֲ�������ȥ������֪A��һ�ָ��۵����ʣ�J��һ�ֺ��ɫ������
��ش��������⣺
(1)A�Ļ�ѧʽΪ ��
(2)H��I��Һ��Ϻ�����Ӧ�����ӷ���ʽ�� ��
G��J�Ļ�ѧ����ʽΪ ��
(3)D����ǡ������һ������ϡ������ú��ʵĻ�ѧ�����ʾ������Һ������ԭ�� ��
(4) ʵ��֤�������ᷢ��������ԭ��Ӧʱ������Խϡ��Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡһ������C��E�Ͻ���һ�����ĺ�ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���һ��Ũ�ȵ�����������Һ�������������Ƶ����ʵ���������ij��������ʵ�����mol���Ĺ�ϵ����ͼ��ʾ���Իش��������⣺
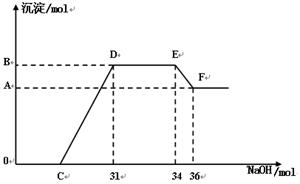
��ͼ��OC��û�г������ɣ��˽η��������ӷ���ʽΪ��_______________________________��
����DE��û�г��������ʵ���û�б仯����˽η�����Ӧ�����ӷ���ʽΪ��_________________________________��
��B���Ӧ�ij�����Ϊ________________ mol,C���Ӧ�ĺ�����Ϊ___________mol��

��ش��������⣺
(1)A�Ļ�ѧʽΪ ��
(2)H��I��Һ��Ϻ�����Ӧ�����ӷ���ʽ�� ��
G��J�Ļ�ѧ����ʽΪ ��
(3)D����ǡ������һ������ϡ������ú��ʵĻ�ѧ�����ʾ������Һ������ԭ�� ��
(4) ʵ��֤�������ᷢ��������ԭ��Ӧʱ������Խϡ��Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡһ������C��E�Ͻ���һ�����ĺ�ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���һ��Ũ�ȵ�����������Һ�������������Ƶ����ʵ���������ij��������ʵ�����mol���Ĺ�ϵ����ͼ��ʾ���Իش��������⣺

��ͼ��OC��û�г������ɣ��˽η��������ӷ���ʽΪ��_______________________________��
����DE��û�г��������ʵ���û�б仯����˽η�����Ӧ�����ӷ���ʽΪ��_________________________________��
��B���Ӧ�ij�����Ϊ________________ mol,C���Ӧ�ĺ�����Ϊ___________mol��
��1��Al2O3��2�֣�
��2��3AlO2����Al3����6H2O=4Al(OH)3�� ��2�֣�4Fe(OH)2+2H2O+O2�T4Fe(OH)3 ��2�֣�
��3��Fe3++3H2O Fe(OH)3+3H+����д��ѧ����ʽ��2�֣�
Fe(OH)3+3H+����д��ѧ����ʽ��2�֣�
��4����H++OH��====H2O ��2�֣�
��NH4++OH��==NH3��H2O��2�֣� ��8 ��1�֣� 7��1�֣�
��2��3AlO2����Al3����6H2O=4Al(OH)3�� ��2�֣�4Fe(OH)2+2H2O+O2�T4Fe(OH)3 ��2�֣�
��3��Fe3++3H2O
 Fe(OH)3+3H+����д��ѧ����ʽ��2�֣�
Fe(OH)3+3H+����д��ѧ����ʽ��2�֣���4����H++OH��====H2O ��2�֣�
��NH4++OH��==NH3��H2O��2�֣� ��8 ��1�֣� 7��1�֣�
����������ͼ�⣬�ؼ�����ͻ�Ƶ㡣A��һ�ָ��۵����ʣ�J��һ�ֺ��ɫ����������A����������J��������������D����������C������B��������E������F���Ȼ�������G��������������H��ƫ�����ƣ�I���Ȼ�����
��1���������Ȼ�ѧʽ��Al2O3��
��2��ƫ�����ƺ��Ȼ���ˮ����ٽ�������������������Ӧ�ķ���ʽ��3AlO2����Al3����6H2O=4Al(OH)3�����������������ȶ������ױ�����������������������ʽ��4Fe(OH)2+2H2O+O2�T4Fe(OH)3 ��
��3���������������������Ȼ������Ȼ���ˮ�⣬��Һ�����ɣ�����ʽ��Fe3++3H2O Fe(OH)3+3H+��
Fe(OH)3+3H+��
��4����ͼ��OC��û�г������ɣ�˵�������ǹ����ģ����Է�Ӧ�ķ���ʽH++OH��====H2O��
����DE��û�г��������ʵ���û�б仯��˵����Һ�л�����NH4+�����Է�Ӧ�ķ���ʽ��NH4++OH��==NH3��H2O��
�۸���ͼ���֪��NH4+���ĵ�����������3mol����˷�Ӧ��ת�Ƶ��ӵ����ʵ�����24mol���ܽ������������ĵ�����������2mol�������������������ĵ�����������6mol�����Խ����������ʵ�����2mol��ʧȥ6mol���ӣ���˸��ݵ��ӵĵ�ʧ�غ��֪���������ʵ����ǣ�24mol��6mol����3��6mol������BB���Ӧ�ij�����8mol������6mol����������������������18mol������C����31mol��18mol��6mol��7mol��
��1���������Ȼ�ѧʽ��Al2O3��
��2��ƫ�����ƺ��Ȼ���ˮ����ٽ�������������������Ӧ�ķ���ʽ��3AlO2����Al3����6H2O=4Al(OH)3�����������������ȶ������ױ�����������������������ʽ��4Fe(OH)2+2H2O+O2�T4Fe(OH)3 ��
��3���������������������Ȼ������Ȼ���ˮ�⣬��Һ�����ɣ�����ʽ��Fe3++3H2O
 Fe(OH)3+3H+��
Fe(OH)3+3H+����4����ͼ��OC��û�г������ɣ�˵�������ǹ����ģ����Է�Ӧ�ķ���ʽH++OH��====H2O��
����DE��û�г��������ʵ���û�б仯��˵����Һ�л�����NH4+�����Է�Ӧ�ķ���ʽ��NH4++OH��==NH3��H2O��
�۸���ͼ���֪��NH4+���ĵ�����������3mol����˷�Ӧ��ת�Ƶ��ӵ����ʵ�����24mol���ܽ������������ĵ�����������2mol�������������������ĵ�����������6mol�����Խ����������ʵ�����2mol��ʧȥ6mol���ӣ���˸��ݵ��ӵĵ�ʧ�غ��֪���������ʵ����ǣ�24mol��6mol����3��6mol������BB���Ӧ�ij�����8mol������6mol����������������������18mol������C����31mol��18mol��6mol��7mol��

��ϰ��ϵ�д�
�����Ŀ