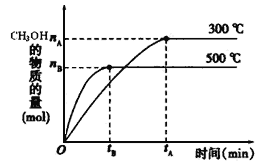
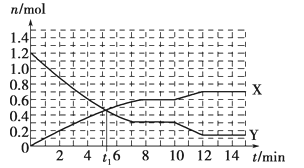
��Ŀ����
����Ŀ��ij��Һ�п��ܺ���Na����Al3����Fe3����Cl����I����![]() ��
��![]() ��ij��ȤС���������ʵ�飺(1)ȡ��������Һ����������ᱵ��Һ����˵ð�ɫ��������Һ(2)��������ɫ�����м�������ϡHNO3�����ֳ�����ȫ�ܽ�(3)��(1)��������Һ�м����������ᣬ������Һ���ɫ,����������ȷ����
��ij��ȤС���������ʵ�飺(1)ȡ��������Һ����������ᱵ��Һ����˵ð�ɫ��������Һ(2)��������ɫ�����м�������ϡHNO3�����ֳ�����ȫ�ܽ�(3)��(1)��������Һ�м����������ᣬ������Һ���ɫ,����������ȷ����
A. ԭ��Һ�п��ܺ���Na����![]()
B. �ɣ�3���ƶ�ԭ��Һ�д���Fe3��
C. ԭ��Һ��һ����I��![]() ��Na��
��Na��
D. ͨ���ڻ�ɫ��Һ�м�����������Һ���Լ���ԭ��Һ���Ƿ����Cl��
���𰸡�C
��������
(1)������ԭ��Һ�м���������ᱵ��Һ���ɰ�ɫ����,���˺�,��ð�ɫ�����м�������ϡ����, ������ȫ�ܽ�,��ð�ɫ�����к���BaCO3��û��BaSO3��(BaSO3����������ΪBaSO4)������ԭ��Һ�к���CO32-��û��SO32-������CO32-��Fe3+��Mg2+���ܴ�������������ԭ��Һ�в�����Fe3+��Mg2+����(1)��������Һ�м�������������,��Һ���ɫ,����������������NO3-����ǿ������,����I-����I2,����ԭ��Һ�к���I-,���������Ӿ������������ܹ������������������Һ�в�����Fe3+��������Һ�ʵ�����֪,ԭ��Һ�п϶�����Na+,��ȷ���Ƿ���Cl-��������Ϸ��������
��(1)������ԭ��Һ�м���������ᱵ��Һ���ɰ�ɫ����,���˺�,��ð�ɫ�����м�������ϡ����, ������ȫ�ܽ�,��ð�ɫ�����к���BaCO3��û��BaSO3��(BaSO3����������ΪBaSO4)������ԭ��Һ�к���CO32-��û��SO32-������CO32-��Fe3+��Mg2+���ܴ�������������ԭ��Һ�в�����Fe3+��Mg2+����(1)��������Һ�м�������������,��Һ���ɫ,����������������NO3-����ǿ������,����I-����I2,����ԭ��Һ�к���I-,���������Ӿ������������ܹ������������������Һ�в�����Fe3+��������Һ�ʵ�����֪,ԭ��Һ�п϶�����Na+,��ȷ���Ƿ���Cl-��
�ۺ����Ϸ�����֪��ԭ��Һ�к���I-��Na+��CO32-��C��ȷ��һ������SO32-��Fe3+��Mg2+��A������B����������֮ǰ�����˴���Cl-,�����ڻ�ɫ��Һ�м�����������Һ����ʹ���Ȼ�����ɫ��������,Ҳ����˵��ԭ��Һ��һ������Cl-,D������
��������������ѡC��

����Ŀ���������ȣ�NOCl���۵㣺��64.5 �棬�е㣺��5.5 �棩��һ�ֻ�ɫ���壬��ˮ��Ӧ����һ������������ֵ��ij����������������Ӧ�ù㷺�������ںϳ���������ý�����м���ȡ�ʵ���ҿ���������һ�������ڳ��³�ѹ�ºϳɡ�
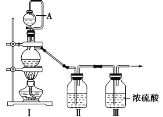
��1������ͬѧ���Ʊ�ԭ����NO��Cl2���Ʊ�װ����ͼ��ʾ��Ϊ�Ʊ�������������壬�����ұ���ȱ�ٵ�ҩƷ��
װ�â� | װ�â� | ||
������ƿ�� | A������ | ||
�Ʊ�������Cl2 | MnO2 | ��______ | ��______ |
�Ʊ�������NO | Cu | ��______ | ��______ |
��2������ͬѧ�Լ���ͬѧ��ȡNO��װ���ԼӸ�������ϼ����Ƶõ�Cl2��ͬ�Ʊ�NOCl��װ����ͼ��ʾ��
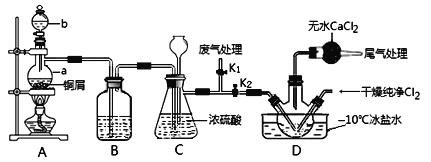
������b������Ϊ________________��
����װ��ʵ��װ�ú�Ӧ��______________��Ȼ������װ��ҩƷ����ʵ��ؼ����������㣺һ�ǽ�Cl2����Dװ�õ�����ƿ�У�����A�з�Ӧ��ʼʱҪ�ȹر�K2����K1����NO����װ�ú��ٹر�K1����K2��������������������Ŀ�Ķ���__________________________________________________��
����Cװ����ѹǿ�����Թ۲쵽��������_________________________��
��װ��D�б���ˮ��������__________________��
��3���������ȣ�NOCl�����ȵIJⶨ���������������ȣ�NOCl����Ʒ13.10g����ˮ�����Ƴ�250mL��Һ��ȡ��25.00mL����K2CrO4��ҺΪָʾ������0.8mol��L-1AgNO3����Һ�ζ����յ㣬���ı���Һ�����Ϊ 22.50mL������֪��Ag2CrO4Ϊש��ɫ���壩
���������ȣ�NOCl����ˮ��Ӧ�Ļ�ѧ����ʽΪ_________________________��
���������ȣ�NOCl������������Ϊ______________________��