��Ŀ����
14�� ϩ��A��һ�������¿�����ͼ�Ŀ�ͼ���з�Ӧ��
ϩ��A��һ�������¿�����ͼ�Ŀ�ͼ���з�Ӧ����֪��A�ĺ˴Ź�������ͼ��ֻ��һ�����շ壮
��1��A�Ľṹ��ʽΪ��CH3��2C=C��CH3��2��
��2����ͼ������ȡ����Ӧ���Ǣڣ�����ţ���
��3����ͼ�Т٢ۢܢ����ڼӳɷ�Ӧ��
��4��C�Ľṹ��ʽΪ��CH3��2CHCCl��CH3��2��
��5��д����ӦD��E�Ļ�ѧ����ʽ����CH3��2CBrCBr��CH3��2+2NaOH$��_{��}^{�Ҵ�}$CH2=C��CH3��C��CH3��=CH2+2NaBr+2H2O��д����ӦE��F�Ļ�ѧ����ʽ��CH2=C��CH3��C��CH3��=CH2+2Br2��CH2BrCBr��CH3��CBr��CH3��CH2Br��
���� A��ϩ����A�ĺ˴Ź�������ͼ��ֻ��һ�����շ壬��A�ṹ��ʽΪ��CH3��2C=C��CH3��2��A�����������ӳɷ�Ӧ����B��B�ṹ��ʽΪ��CH3��2CHCH��CH3��2��B�����ڹ��������·���ȡ����Ӧ����C����CΪ��CH3��2CHCCl��CH3��2��A�����ӳɷ�Ӧ����C��A���巢���ӳɷ�Ӧ����D��D�ṹ��ʽΪ��CH3��2CBrCBr��CH3��2��D������ŨNaOH���Ҵ���Һ�м��ȷ�����ȥ��Ӧ����E��E�ṹ��ʽΪCH2=C��CH3��C��CH3��=CH2��E���巢���ӳɷ�Ӧ����F��F�ṹ��ʽΪCH2BrCBr��CH3��CBr��CH3��CH2Br���ݴ˷������
��� �⣺A��ϩ����A�ĺ˴Ź�������ͼ��ֻ��һ�����շ壬��A�ṹ��ʽΪ��CH3��2C=C��CH3��2��A�����������ӳɷ�Ӧ����B��B�ṹ��ʽΪ��CH3��2CHCH��CH3��2��B�����ڹ��������·���ȡ����Ӧ����C����CΪ��CH3��2CHCCl��CH3��2��A�����ӳɷ�Ӧ����C��A���巢���ӳɷ�Ӧ����D��D�ṹ��ʽΪ��CH3��2CBrCBr��CH3��2��D������ŨNaOH���Ҵ���Һ�м��ȷ�����ȥ��Ӧ����E��E�ṹ��ʽΪCH2=C��CH3��C��CH3��=CH2��E���巢���ӳɷ�Ӧ����F��F�ṹ��ʽΪCH2BrCBr��CH3��CBr��CH3��CH2Br��
��1��A�Ľṹ��ʽΪ��CH3��2C=C��CH3��2���ʴ�Ϊ����CH3��2C=C��CH3��2��
��2����ͼ������ȡ����Ӧ���Ǣڣ��ʴ�Ϊ���ڣ�
��3����ͼ�Т٢ۢܢ����ڼӳɷ�Ӧ���ʴ�Ϊ���ӳɣ�
��4��C�Ľṹ��ʽΪ��CH3��2CHCCl��CH3��2���ʴ�Ϊ����CH3��2CHCCl��CH3��2��
��5��D�ṹ��ʽΪ��CH3��2CBrCBr��CH3��2��D������ŨNaOH���Ҵ���Һ�м��ȷ�����ȥ��Ӧ����E��E�ṹ��ʽΪCH2=C��CH3��C��CH3��=CH2����D��E�Ļ�ѧ����ʽ����CH3��2CBrCBr��CH3��2+2NaOH$��_{��}^{�Ҵ�}$CH2=C��CH3��C��CH3��=CH2+2NaBr+2H2O��E�ṹ��ʽΪCH2=C��CH3��C��CH3��=CH2��E���巢���ӳɷ�Ӧ����F��F�ṹ��ʽΪCH2BrCBr��CH3��CBr��CH3��CH2Br����Ӧ����ʽΪCH2=C��CH3��C��CH3��=CH2+2Br2��CH2BrCBr��CH3��CBr��CH3��CH2Br��
�ʴ�Ϊ����CH3��2CBrCBr��CH3��2+2NaOH$��_{��}^{�Ҵ�}$CH2=C��CH3��C��CH3��=CH2+2NaBr+2H2O��CH2=C��CH3��C��CH3��=CH2+2Br2��CH2BrCBr��CH3��CBr��CH3��CH2Br��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ƶ���������ȷ�����ż������ʹ�ϵ�ǽⱾ��ؼ������÷�Ӧ���������ʽṹ�����ƶϣ���Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | Na+��K+��MnO4-��NO3- | B�� | ClO-��I-��NH4+��Ba2+ | ||
| C�� | Na+��AlO2-��K+��HCO3- | D�� | Al3+��K+��SO42-��NO3- |
| A�� | ��ij��Һ�м���NaOH��Һ�����ȣ�������������ʹʪ�����ɫʯ����ֽ��죬����Һ��һ������NH4+ | |
| B�� | ��ij��Һ�м���BaCl2��Һ�����ɰ�ɫ��������ó����м���ϡ���ᣬ������������ʹ����ʯ��ˮ����ǣ���ԭ��Һ�п��ܺ���SO32- | |
| C�� | ��ij��Һ�м��������ữ��BaCl2��Һ���а�ɫ�������ɣ������Һ��һ������SO42- | |
| D�� | ��ij��Һ�м���2�λ�ɫK3[Fe��CN��6]��Һ��������ɫ�����������Һ��һ������Fe2+ |
| A�� | ����NH4NO3������ײ���¿��ܷ�����ը | |
| B�� | ��ʳƷ���з���ʢ�й轺����С�����ɷ�ֹʳ���ܳ� | |
| C�� | ��������������Ʊ�̫���ܵ�ذ壬�����������������ά | |
| D�� | ̼�����ƿ���������θ����� |
| A�� | 22.4LCO2����NA��CO2���� | B�� | 0.1NA��H2SO4���ӵ�����Ϊ9.8�� | ||
| C�� | 10gCaCO3����NA��Cԭ�� | D�� | 2molCl2����2NA��Clԭ�� |
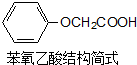 ��������һ�־��в���������ʳ�����ϣ��ǻ�������뱽�����ᷢ��������Ӧ�IJ��
��������һ�־��в���������ʳ�����ϣ��ǻ�������뱽�����ᷢ��������Ӧ�IJ�� 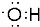 ��
��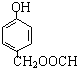 ��
��