��Ŀ����
�����ڿƼ���������Ӧ�ù㷺��
��1����ҵ�ϳ�����ʯ�Һ�������Ӧ��ȡƯ�ۣ���ѧ����ʽ�� ��
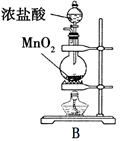
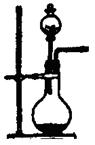
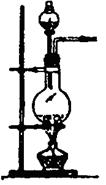
��2��ʵ������MnO2��Ũ���ᷴӦ��ȡ������ԭ�����£�MnO2 + 4HCl MnCl2 + Cl2��+ 2H2O
MnCl2 + Cl2��+ 2H2O
������ȡ11.2 L Cl2����״������������Ӧ����MnO2������Ϊ______g��
����ƽ���ƶ�ԭ�����Ϳ����ű���ʳ��ˮ���ռ�������ԭ�� ������ϱ�Ҫ�Ļ�ѧ���P���ֻش�
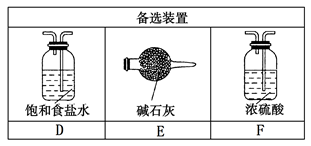
���Ʊ�����ʱ������NaOH��Һ����β���������Լ�Ҳ������������������____������ĸ����
a. KI��Һ b. FeCl2��Һ c. KCl��Һ
д����ѡ��������Լ���Cl2��Ӧ�����ӷ���ʽ��_______��
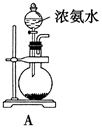
��Ҳ����Ũ��ˮ����������ͬʱ����һ������Ⱦ�����壬��Ӧ�Ļ�ѧ����ʽ��_______��
��1����ҵ�ϳ�����ʯ�Һ�������Ӧ��ȡƯ�ۣ���ѧ����ʽ�� ��
��2��ʵ������MnO2��Ũ���ᷴӦ��ȡ������ԭ�����£�MnO2 + 4HCl
 MnCl2 + Cl2��+ 2H2O
MnCl2 + Cl2��+ 2H2O������ȡ11.2 L Cl2����״������������Ӧ����MnO2������Ϊ______g��
����ƽ���ƶ�ԭ�����Ϳ����ű���ʳ��ˮ���ռ�������ԭ�� ������ϱ�Ҫ�Ļ�ѧ���P���ֻش�
���Ʊ�����ʱ������NaOH��Һ����β���������Լ�Ҳ������������������____������ĸ����
a. KI��Һ b. FeCl2��Һ c. KCl��Һ
д����ѡ��������Լ���Cl2��Ӧ�����ӷ���ʽ��_______��
��Ҳ����Ũ��ˮ����������ͬʱ����һ������Ⱦ�����壬��Ӧ�Ļ�ѧ����ʽ��_______��
��1��2Ca(OH)2+2Cl2=CaCl2+Ca(ClO)2+2H2O
��2����43.5����Cl2����ˮ��ˮ��Ӧ��Cl2+H2O
 HClO+HCl�����͵�ʳ��ˮ�д��ڴ�����Cl-��ƽ�������ƶ���ʹCl2���ܽ�ȼ�С�����ԣ������ű���ʳ��ˮ���ռ�Cl2����ab ����������ԭ��Ӧ���ӷ���ʽΪ�� Cl2+2I-=2 Cl-+I2��Cl2+2Fe2+= 2Fe3++2Cl-����3Cl2+8NH3=6NH4Cl+N2����3Cl2+2NH3=6HCl+N2��д��һˮ�ϰ�Ҳ�У���
HClO+HCl�����͵�ʳ��ˮ�д��ڴ�����Cl-��ƽ�������ƶ���ʹCl2���ܽ�ȼ�С�����ԣ������ű���ʳ��ˮ���ռ�Cl2����ab ����������ԭ��Ӧ���ӷ���ʽΪ�� Cl2+2I-=2 Cl-+I2��Cl2+2Fe2+= 2Fe3++2Cl-����3Cl2+8NH3=6NH4Cl+N2����3Cl2+2NH3=6HCl+N2��д��һˮ�ϰ�Ҳ�У������������
��1����ҵ������ʯ�Һ�������Ӧ��ȡƯ�۵Ļ�ѧ����ʽΪ��2Cl2+2Ca(OH)2��CaCl2+Ca(ClO)2+2H2O��
��2������MnO2������ΪX
MnO2 + 4HCl
 MnCl2 + Cl2��+ 2H2O
MnCl2 + Cl2��+ 2H2O87g 22.4L
X 11.2L
X=43.5g
��Cl2��ˮ��Һ�д��ڣ�Cl2+H2O
 HClO+HCl�����͵�ʳ��ˮ�д��ڴ�����Cl-���������������ܽ�,ʹ��ѧƽ�����ƣ��������ܽ�ȼ�С����Cl2+2I-=2 Cl-+I2��Cl2+2Fe2+= 2Fe3++2Cl- ��������ǿ���ԡ������л�ԭ�ԣ����߷���������ԭ��Ӧ����ѧ����ʽΪ3Cl2+8NH3=6NH4Cl+N2����3Cl2+2NH3=6HCl+N2��
HClO+HCl�����͵�ʳ��ˮ�д��ڴ�����Cl-���������������ܽ�,ʹ��ѧƽ�����ƣ��������ܽ�ȼ�С����Cl2+2I-=2 Cl-+I2��Cl2+2Fe2+= 2Fe3++2Cl- ��������ǿ���ԡ������л�ԭ�ԣ����߷���������ԭ��Ӧ����ѧ����ʽΪ3Cl2+8NH3=6NH4Cl+N2����3Cl2+2NH3=6HCl+N2��
��ϰ��ϵ�д�
�����Ŀ




 2KCl + 2MnCl2 + 5Cl2 �� + 8H2O
2KCl + 2MnCl2 + 5Cl2 �� + 8H2O