��Ŀ����
����Ŀ������ѧѡ3�������ʽṹ������������ �ϳɵ�������Ԫ����[La0.8Cu0.2Ni1-xMxO3(M �ֱ�ΪMn��Fe��Co)]����ʹ����β����NO��CO������Ӧ������β����Ⱦ��ͬʱ�ɴ���ؽ������������ش��������⣺
�ϳɵ�������Ԫ����[La0.8Cu0.2Ni1-xMxO3(M �ֱ�ΪMn��Fe��Co)]����ʹ����β����NO��CO������Ӧ������β����Ⱦ��ͬʱ�ɴ���ؽ������������ش��������⣺
(1)Mn2+�ĺ�������Ų�ʽΪ��________________���䵥������Ϊ_________________��
(2)C��N��O��Mn�縺���ɴ�С��˳����___________��
(3)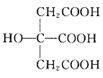 Ҳ�dz������壬���в�ȡsp2�ӻ���̼ԭ�Ӻ�sp3�ӻ���̼ԭ�Ӹ�����Ϊ_____��
Ҳ�dz������壬���в�ȡsp2�ӻ���̼ԭ�Ӻ�sp3�ӻ���̼ԭ�Ӹ�����Ϊ_____��
(4)��ɫ����KFe(��)x[Fe(��)(CN)6]�ɻ����ؽ����ж���x=_______���������в����ڵ���������_____��
A�����»��� B�����Ӽ� C������ D������ E.���
(5)����Ԫ���ܵ��������������������ȫ������ȩ(HCHO)����ȩ���ӵ����幹��Ϊ_____����ȩ������Ϊ������״�(CH3OH)ΪҺ���ԭ����________________________________ ��
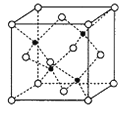
(6)����Ԫ��Mn��Ԫ��Se�γɵ�ij����������������ϵ���侧���ṹ��ͼ��ʾ������(��ΪSe��![]() ΪMn)���û�����Ļ�ѧʽΪ________��Se����λ��Ϊ______��Mn��Se��Ħ�������ֱ�ΪM1g/mol��M2g/mol���þ�����ܶ�Ϊ��g/cm3,��Mn��Se���ļ���Ϊ_____________nm(�������ʽ)��
ΪMn)���û�����Ļ�ѧʽΪ________��Se����λ��Ϊ______��Mn��Se��Ħ�������ֱ�ΪM1g/mol��M2g/mol���þ�����ܶ�Ϊ��g/cm3,��Mn��Se���ļ���Ϊ_____________nm(�������ʽ)��
���𰸡�1s22s22p63s23p63d5����[Ar]3d5 5 O��N��C��Mn 1:1 1 AE ƽ�������� �״������γɷ��Ӽ���� MnSe 4 ![]()
��������
(1)����25��Ԫ�أ�Mn2+�ĺ�������Ų�ʽΪ1s22s22p63s23p63d5������3d5�е�5�����ӷֲ���5��ԭ�ӹ���У���˵�������Ϊ5���ʴ�Ϊ��1s22s22p63s23p63d5��5��
(2)Ԫ�صĽ�����Խǿ���縺����ֵԽС���ǽ�����Խǿ���縺����ֵԽ��C��N��O��Mn�縺���ɴ�С��˳����O��N��C��Mn���ʴ�Ϊ��O��N��C��Mn��
(3)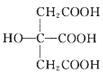 Ҳ�dz������壬����C=O�е�̼ԭ�Ӳ�ȡsp2�ӻ�����3��������̼ԭ�Ӳ���sp3�ӻ�����3����������Ϊ1:1���ʴ�Ϊ��1:1��
Ҳ�dz������壬����C=O�е�̼ԭ�Ӳ�ȡsp2�ӻ�����3��������̼ԭ�Ӳ���sp3�ӻ�����3����������Ϊ1:1���ʴ�Ϊ��1:1��
(4)�����������ϼ۴�����Ϊ0��KFe(III) x[Fe(II)(CN)6]�д���(+1)+(+3)��x+(+2)+(-1)��6=0�����x=1�������������������д��ڵ������������Ӽ���������CN-�д����������Լ���λ���������ڷ��»������������ѡAE���ʴ�Ϊ��1��AE��
(5)��ȩ�����е�̼ԭ��3��ԭ��������û�й¶Ե��ӣ�����sp2�ӻ������幹��Ϊƽ�����������״������γɷ��Ӽ����������ȩ���ܣ���˼�ȩ������Ϊ������״�(CH3OH)ΪҺ�壬�ʴ�Ϊ��ƽ�������Σ��״������γɷ��Ӽ������
(6)���ݾ����ṹͼ��Seԭ�Ӹ���Ϊ8��![]() +6��
+6��![]() =4��Mnԭ�Ӹ���=4���û�����Ļ�ѧʽΪMnSe��ÿ��Se��Χ��4��Mnԭ�Ӿ����������ȣ�Se����λ��Ϊ4��1mol����������Ϊ(4M1+4M2)g���辧���ı߳�Ϊxcm��1mol���������ΪNA��x3cm3����x=
=4��Mnԭ�Ӹ���=4���û�����Ļ�ѧʽΪMnSe��ÿ��Se��Χ��4��Mnԭ�Ӿ����������ȣ�Se����λ��Ϊ4��1mol����������Ϊ(4M1+4M2)g���辧���ı߳�Ϊxcm��1mol���������ΪNA��x3cm3����x=![]() cm��Mn��Se���ļ���Ϊ������Խ��߳��ȵ�
cm��Mn��Se���ļ���Ϊ������Խ��߳��ȵ�![]() ����Mn��Se���ļ���=
����Mn��Se���ļ���=![]() ��
��![]() cm=
cm=![]() ��
��![]() ��107nm���ʴ�Ϊ��MnSe��4��
��107nm���ʴ�Ϊ��MnSe��4��![]() ��
��![]() ��107��
��107��

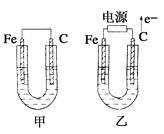
����Ŀ����ͼ��ʾ��ʵ�飬�ܴﵽʵ��Ŀ�ĵ���
A | B | C | D |
|
|
|
|
��֤��ѧ��ת��Ϊ���� | �о������Ի�ѧ��Ӧ���ʵ�Ӱ�� | ʵ�����ư��� | ��֤�ǽ����ԣ�Cl>C>Si |
A. AB. BC. CD. D
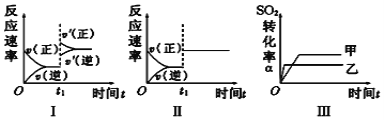
����Ŀ�����ܱ����м���2.0molSO2��1.0molO2��һ�������·������·�Ӧ2SO2��O2![]() 2SO3����Ӧ�ﵽƽ������ʵ����ʵ�������Ϊ( )
2SO3����Ӧ�ﵽƽ������ʵ����ʵ�������Ϊ( )
n(SO2)/mol | n(O2)/mol | n(SO3)/mol | |
A | 2.0 | 1.0 | 0 |
B | 1.0 | 0.8 | 1.0 |
C | 0.20 | 0.10 | 1.80 |
D | 0 | 0 | 2.0 |
A. AB. BC. CD. D