��Ŀ����
��2010?�Ϻ���Na2SO3?7H2O��ʳƷ��ҵ�г��õ�Ư�������������ͷ�������Na2SO3��30��ʱ���ܽ��Ϊ35.5g/100gH2O��
1������30��ʱNa2SO3������Һ��Na2SO3�����������أ�������2λС����
2������30��ʱ271g Na2SO3������Һ��ˮ��������
3����30���Na2SO3������Һ271g��ȴ��10�棬����Na2SO3?7H2O����79.5g������10��ʱNa2SO3��ˮ�е��ܽ�ȣ�
1������30��ʱNa2SO3������Һ��Na2SO3�����������أ�������2λС����
2������30��ʱ271g Na2SO3������Һ��ˮ��������
3����30���Na2SO3������Һ271g��ȴ��10�棬����Na2SO3?7H2O����79.5g������10��ʱNa2SO3��ˮ�е��ܽ�ȣ�
������1���������ʵ���������=
��Na2SO3��30��ʱ���ܽ�������㱥����Һ��Na2SO3�����������أ�
2������Na2SO3��30��ʱ���ܽ��������271g Na2SO3������Һ��ˮ��������
3������Na2SO3?7H2O�����ԭ��Һ�����ʵ��������10���DZ�����Һ�����ʵ��������ܼ��������������10��ʱNa2SO3��ˮ�е��ܽ�ȣ���100gˮ���ܽ� Na2SO3��������
| ���ʵ����� |
| ��Һ������ |
2������Na2SO3��30��ʱ���ܽ��������271g Na2SO3������Һ��ˮ��������
3������Na2SO3?7H2O�����ԭ��Һ�����ʵ��������10���DZ�����Һ�����ʵ��������ܼ��������������10��ʱNa2SO3��ˮ�е��ܽ�ȣ���100gˮ���ܽ� Na2SO3��������
����⣺1������Na2SO3���ܽ�ȣ��䱥����Һ������Ϊ35.5g���ܼ�Ϊ100g����Һ������=100+35.5=135.5g��
��أ�Na2SO3��=
��0.26��
2��271g������Һ�У������京�е��ܼ�Ϊx��
Na2SO3��30��ʱ���ܽ��Ϊ35.5g/100gH2O��
��
=
��
��֮�ã�x=200��g����
3����ȴ��Һ����������79.5g�������侧��Na2SO3?7H2O����ɣ����к���ˮ����������Ϊ��
m��Na2SO3��=79.5g��
=39.75g
m��H2O��=79.5g-39.75g=39.75g
10��ʱNa2SO3������Ϊ271-200-39.75=31.25g
10��ʱH2O������Ϊ200-39.75=160.25g
����10��ʱNa2SO3���ܽ��Ϊy
=
��
���y=19.5g��
��1��30��ʱNa2SO3������Һ��Na2SO3����������Ϊ0.26��
2��30��ʱ271g Na2SO3������Һ��ˮ������Ϊ200g��
3��10��ʱNa2SO3��ˮ�е��ܽ��Ϊ19.5g��
��أ�Na2SO3��=
| 35.5g |
| 135.5g |
2��271g������Һ�У������京�е��ܼ�Ϊx��
Na2SO3��30��ʱ���ܽ��Ϊ35.5g/100gH2O��
��
| 100g |
| 135.5g |
| x |
| 271g |
��֮�ã�x=200��g����
3����ȴ��Һ����������79.5g�������侧��Na2SO3?7H2O����ɣ����к���ˮ����������Ϊ��
m��Na2SO3��=79.5g��
| 126 |
| 126+126 |
m��H2O��=79.5g-39.75g=39.75g
10��ʱNa2SO3������Ϊ271-200-39.75=31.25g
10��ʱH2O������Ϊ200-39.75=160.25g
����10��ʱNa2SO3���ܽ��Ϊy
| 100g |
| y |
| 160.25g |
| 31.25g |
���y=19.5g��
��1��30��ʱNa2SO3������Һ��Na2SO3����������Ϊ0.26��
2��30��ʱ271g Na2SO3������Һ��ˮ������Ϊ200g��
3��10��ʱNa2SO3��ˮ�е��ܽ��Ϊ19.5g��
���������⿼������Һ�������������ܼ����������ܽ�ȵȻ�ѧ����֪ʶ��Ҫ�����ܽ�ȵĸ��

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ
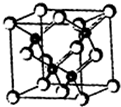 2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������lnGaN���������أ�Ϊ������֪������ͬ����һ���ڵ�Ԫ�أ��黯�صľ����ṹ��ͼ���Իش�
2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������lnGaN���������أ�Ϊ������֪������ͬ����һ���ڵ�Ԫ�أ��黯�صľ����ṹ��ͼ���Իش�
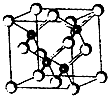 ��2011?�Ͼ�ģ�⣩2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������InGaN���������أ�Ϊ�����黯�ص�Ʒ���ṹ��ͼ���Իش�
��2011?�Ͼ�ģ�⣩2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������InGaN���������أ�Ϊ�����黯�ص�Ʒ���ṹ��ͼ���Իش�