��Ŀ����
��9�֣�(1)����д���пհ״���ʵ������Ҫ����500mL0.2mol/L����������Һ��ʵ�鲽������У�
| A������ƽ�ϳƳ�___________g�����ƹ��壬���������ձ��������������ˮ�ܽ⡣ |
| B���ѵõ�����ҺС�ĵ�����__________ע��________mL������ƿ�С� |
| C������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ��ҺҲС��ת������ƿ�С� |
| D������������ƿ�м�����ˮ��Һ���̶�l��2cm��������_______________С�ĵμ�����ˮ����Һ��Һ��ײ���̶���ˮƽ���С� |
F������õ���Һ�����Լ�ƿ�У����ϱ�ǩ����ϴ������ƿ��
(2) ���������ʹ������ҺŨ��ƫ�ߵ���___________(�����)��
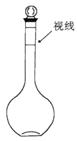
a��ijͬѧ����ʱ�۲�Һ����������ͼ��ʾ
b��û�н��������IJ�������C
c��������ˮʱ�����������˿̶���
d��������������
e������ƿ��ǰ�ڱ�մ��ˮ��
��9�֣���1��A��14.2 B�������� 500 D����ͷ�ι� ��2��ad
����

��ϰ��ϵ�д�
�����Ŀ
A��B��C��D��E��F��G�����ɶ�����Ԫ�ع��ɵ����ӣ����Ƕ���10�����ӣ��ṹ�ص����±���
����д���пհף�
��1��A���ӵĽṹʾ��ͼΪ______��D���ӵĵ���ʽΪ______��G���ӵĻ�ѧʽΪ______��
��2��BC��EC2�ļ���ǿ��ΪBC______EC2�����������=����
��3�����������£�F��C����Һ�з�Ӧ�����ӷ���ʽΪ______ NH3��+H2O
| ���Ӵ��� | A | B | C | D | E | F | G |
| ԭ�Ӻ˸��� | ���� | ���� | ˫�� | �ĺ� | ���� | ��� | ��� |
| ����� | 1+ | 1- | 2+ | 1+ |
��1��A���ӵĽṹʾ��ͼΪ______��D���ӵĵ���ʽΪ______��G���ӵĻ�ѧʽΪ______��
��2��BC��EC2�ļ���ǿ��ΪBC______EC2�����������=����
��3�����������£�F��C����Һ�з�Ӧ�����ӷ���ʽΪ______ NH3��+H2O
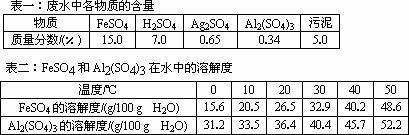

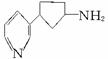
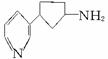 ��������Ŷ����ܷ����Ļ�ѧ��Ӧ��____________(��д�������)��
��������Ŷ����ܷ����Ļ�ѧ��Ӧ��____________(��д�������)��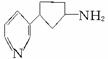
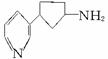 ��������Ŷ����ܷ����Ļ�ѧ��Ӧ��____________(��д�������)��
��������Ŷ����ܷ����Ļ�ѧ��Ӧ��____________(��д�������)��